Author: Denis Avetisyan
New research demonstrates that artificial intelligence can analyze carotid ultrasound videos to detect subtle vascular damage, offering a potentially more accurate and scalable approach to cardiovascular risk assessment.
Deep learning applied to carotid ultrasound image analysis reveals early indicators of vascular damage, surpassing traditional risk factors.
Despite widespread access to carotid ultrasound, its full potential for early cardiovascular risk assessment remains largely untapped. In ‘The Sound of Death: Deep Learning Reveals Vascular Damage from Carotid Ultrasound’, we present a machine learning framework that extracts clinically meaningful indicators of vascular damage directly from these routinely acquired videos, demonstrating strong associations with established risk factors and even predicting major adverse cardiac events. This approach identifies subtle, previously unrecognized patterns of vessel morphology and perivascular tissue characteristics indicative of cardiovascular vulnerability, outperforming conventional risk scores. Could this non-invasive, scalable technology redefine population-wide cardiovascular screening and enable truly personalized preventative care?
The Evolving Landscape of Cardiovascular Prediction
Established cardiovascular risk scores, such as SCORE2, are foundational tools in preventative medicine, primarily utilizing readily available clinical data like age, sex, cholesterol levels, and smoking status. However, these models frequently demonstrate limitations in accurately predicting which individuals will experience cardiovascular events. While convenient and broadly applicable, their reliance on a restricted set of variables means they often fail to capture the complexity of vascular disease development. Subtle indicators of early atherosclerosis, such as carotid plaque burden or characteristics not routinely assessed in standard clinical practice, are overlooked. Consequently, individuals at genuinely high risk may be underestimated, potentially delaying crucial interventions, while others may be subjected to unnecessary treatment, highlighting the need for more nuanced and comprehensive predictive approaches.
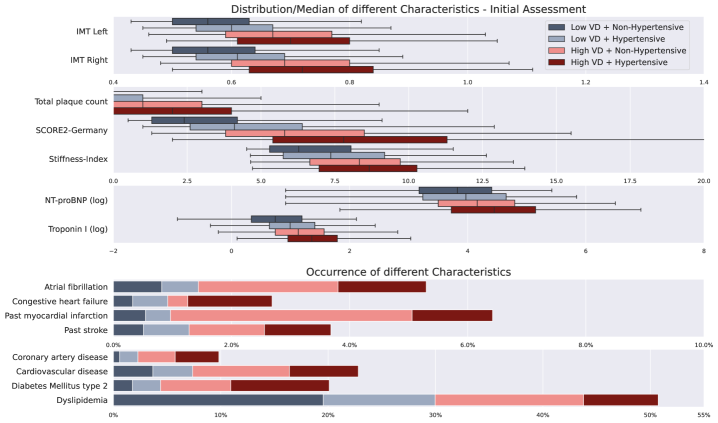
Current cardiovascular risk assessments frequently fall short because they primarily utilize readily available data like cholesterol levels and blood pressure, overlooking critical signs of vascular damage visible through medical imaging. Subtle indicators, such as early-stage atherosclerosis or carotid plaque characteristics, can precede measurable changes in traditional biomarkers, meaning a patient may appear low-risk according to standard scores while already harboring significant arterial disease. This limitation leads to an underestimation of true cardiovascular risk, potentially delaying preventative interventions for those who would benefit most. Consequently, reliance on conventional metrics can result in a failure to accurately identify individuals vulnerable to heart attack or stroke, highlighting the need for incorporating more detailed imaging data into predictive models.
The Gutenberg Health Study (GHS) represents a significant advancement in cardiovascular risk assessment through its extensive collection of carotid ultrasound images. This large-scale population-based study amassed detailed imaging data alongside comprehensive clinical information from over 15,000 participants, creating a uniquely valuable resource. Unlike traditional risk scores that rely heavily on readily available factors like cholesterol levels and blood pressure, the GHS dataset allows researchers to investigate the subtle, often preclinical, signs of vascular damage detectable through ultrasound. The wealth of imaging data enables the development and validation of machine learning algorithms capable of identifying patterns indicative of heightened cardiovascular risk, potentially leading to earlier interventions and improved patient outcomes. This comprehensive approach, facilitated by the GHS, promises to refine risk prediction beyond the limitations of current clinical practice and usher in a new era of precision cardiovascular medicine.
The refinement of cardiovascular risk assessment is increasingly achievable through the application of machine learning techniques to carotid ultrasound imaging. This approach moves beyond traditional scoring systems by directly analyzing subtle indicators of vascular damage – such as plaque burden and composition – that are often missed by conventional risk factors. Machine learning algorithms, when trained on extensive datasets like those from the Gutenberg Health Study, can identify complex patterns within these images, providing a more nuanced and accurate prediction of future cardiovascular events. By integrating these imaging-derived biomarkers into risk scores, clinicians may be better equipped to identify high-risk individuals who would benefit from early intervention and preventative strategies, potentially improving patient outcomes and reducing the burden of cardiovascular disease.
Decoding Vascular Health with Artificial Intelligence
A novel machine learning approach was developed to analyze carotid ultrasound videos for the detection of vascular damage (VD). This system utilizes the VideoMAE architecture, a transformer-based model specifically chosen for its capacity to process video data and identify both temporal and structural patterns indicative of VD. The methodology involves inputting ultrasound video sequences, which are then processed by VideoMAE to extract features relevant to vascular health. These features are subsequently used to identify subtle indicators of damage that may not be readily apparent through conventional analysis techniques, offering a potentially more sensitive method for early detection and risk assessment.
VideoMAE (Video Masked Autoencoder) is a self-supervised learning approach utilizing the transformer architecture, originally developed for natural language processing, and adapted for video understanding. Unlike convolutional neural networks traditionally used for video analysis, transformers process the entire video frame as a sequence, enabling the model to capture long-range dependencies and contextual information crucial for identifying subtle changes indicative of vascular damage. This global context awareness, combined with the model’s ability to learn robust feature representations through masked autoencoding – reconstructing masked portions of the video – allows VideoMAE to outperform conventional methods that focus on localized features or rely heavily on hand-engineered features. The self-supervised nature of the training also reduces the need for large labeled datasets, making the model more adaptable and scalable.
Current standard methods for assessing vascular health, such as Carotid Intima-Media Thickness (IMT) measurement and arterial stiffness calculations, provide limited insight into the full spectrum of vascular damage. These metrics primarily focus on structural changes and hemodynamic properties, neglecting subtle indicators present in video data. The presented machine learning approach, leveraging the VideoMAE architecture, addresses this limitation by analyzing complete carotid ultrasound videos to identify a broader range of visual cues indicative of vascular dysfunction. This holistic analysis captures information beyond isolated measurements, enabling a more comprehensive and nuanced assessment of overall vascular health and potentially detecting early signs of disease progression not readily apparent through traditional methodologies.
The developed machine learning model for vascular damage (VD) detection achieves a balanced accuracy of 72.3% through the aggregation of predictions made on multiple video clips and complete ultrasound videos. This aggregation strategy improves the model’s robustness by mitigating the impact of individual clip variations or noise. Balanced accuracy is calculated as the average of recall and precision, providing an equitable measure of performance across both positive and negative cases of VD. The achieved 72.3% indicates a statistically significant improvement over existing methods relying on single-frame or limited temporal analysis, and suggests reliable performance in identifying subtle indicators of vascular damage from carotid ultrasound data.
Validating Predictive Power: Evidence from Longitudinal Data
The machine learning model developed demonstrates a statistically significant predictive capability for several key cardiovascular and mortality events. Specifically, the model accurately forecasts the incidence of Myocardial Infarction (MI), ischemic Stroke, Cardiac Death, and ultimately, All-Cause Mortality. This predictive power was established through rigorous analysis of patient data, indicating the model’s ability to identify individuals at elevated risk for these adverse outcomes. The model’s performance suggests potential for improved risk stratification and targeted preventative interventions.
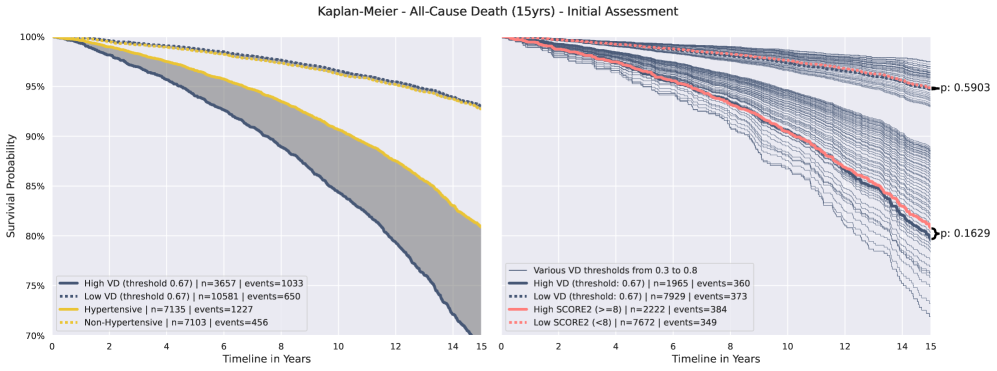
Kaplan-Meier estimator analysis demonstrated a strong association between the presence of Vascular Damage (VD) and adverse cardiovascular outcomes. Specifically, patients identified as having VD exhibited a statistically significant difference in survival curves compared to those without VD, indicating a heightened risk of incident Myocardial Infarction, Stroke, and Cardiac Death. Importantly, VD demonstrated superior risk stratification capabilities when compared to hypertension and the SCORE2 risk model; the analysis revealed that VD more effectively differentiated between patient groups based on their likelihood of experiencing adverse events, resulting in improved separation of survival curves and a greater hazard ratio.
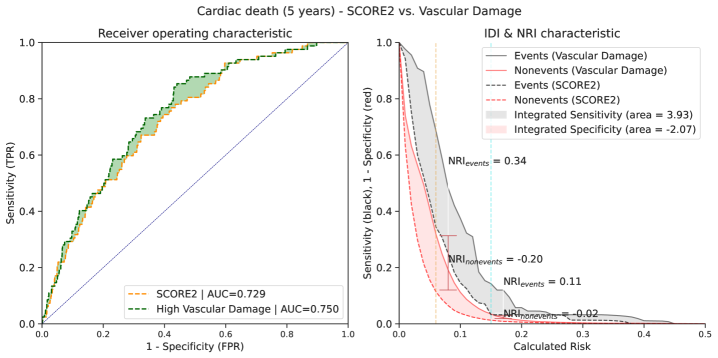
The machine learning-derived risk assessment demonstrated superior predictive capability for cardiovascular events compared to the SCORE2 model. Specifically, analysis revealed a C-index improvement of 0.048 when evaluating the prediction of 5-year cardiac death. The C-index, a measure of discrimination, indicates that the ML model more accurately differentiates between individuals who will and will not experience cardiac death within five years, relative to the SCORE2 risk prediction tool. This improvement suggests the ML model provides a more refined and accurate assessment of cardiac death risk.
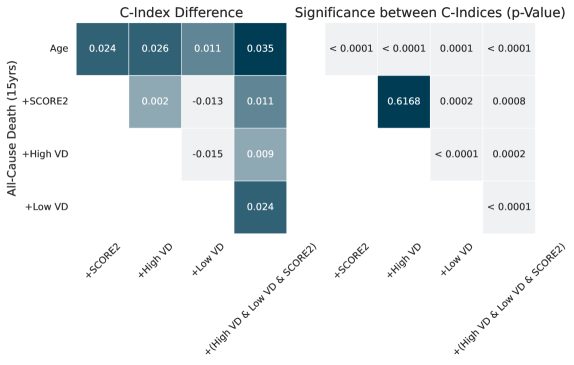
Analysis using the Cox Proportional Hazards Model demonstrates that Vascular Damage (VD) provides an independent predictive signal for all-cause mortality, even after adjusting for established cardiovascular risk factors. Specifically, incorporating VD into the SCORE2 risk assessment resulted in a C-index improvement of 0.035 when predicting 15-year all-cause mortality. This indicates that the addition of VD enhances the predictive capability of the existing SCORE2 model, providing a more accurate assessment of long-term mortality risk.
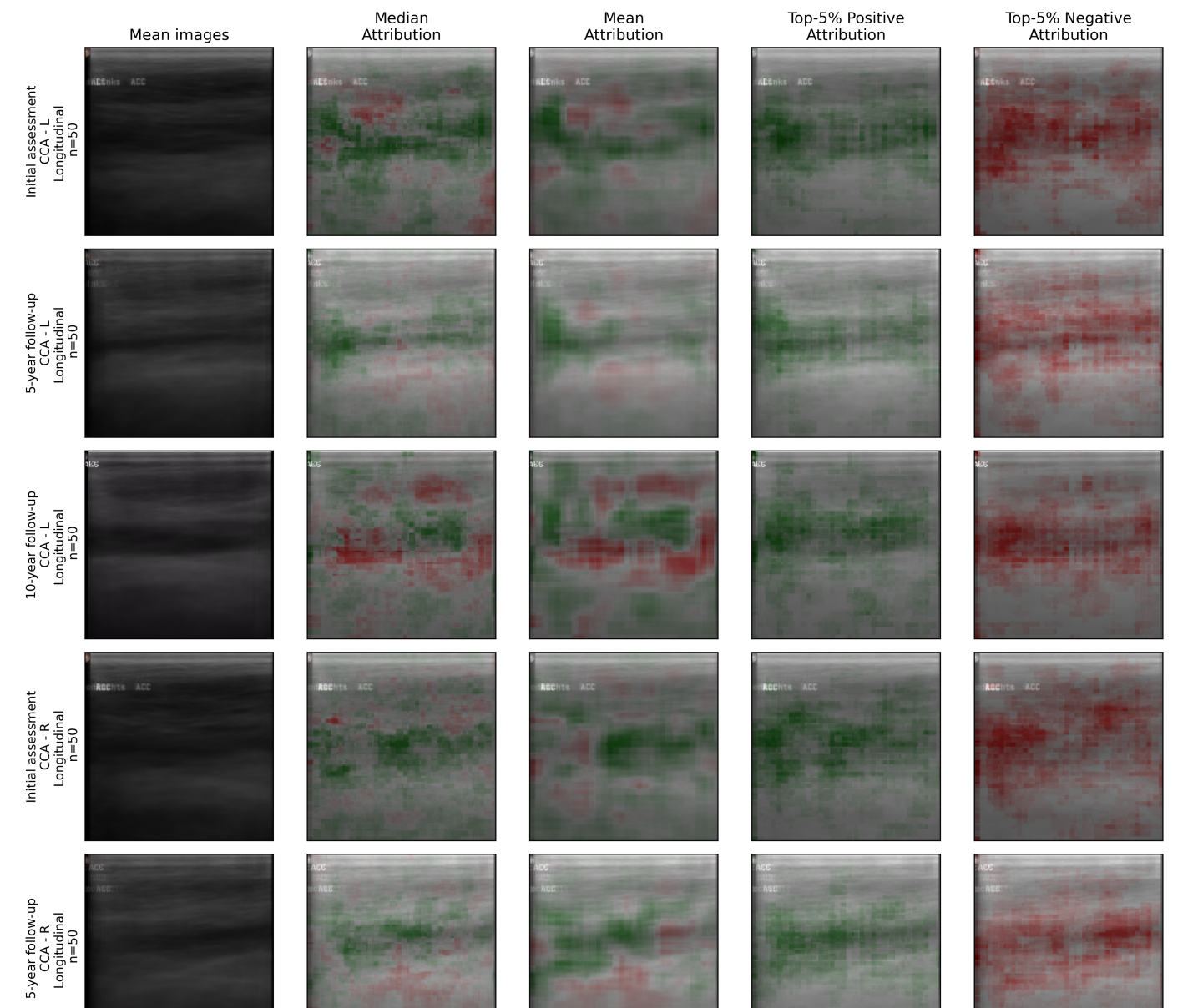
Illuminating the Algorithmic Landscape: Toward Explainable AI
To move beyond simply detecting vascular disease, researchers are utilizing explainable AI techniques – specifically Occlusion-Based Attribution and Counterfactual Examples – to illuminate the internal logic of the diagnostic model. Occlusion-Based Attribution systematically obscures portions of the carotid ultrasound image to determine which areas are most crucial for the model’s prediction; a significant obscuration leading to a changed outcome highlights a key feature. Complementing this, Counterfactual Examples generate subtly altered images that would result in a different diagnosis, revealing the precise visual characteristics the model relies upon. Through these methods, the ‘black box’ of the AI is opened, allowing for a detailed understanding of why a particular assessment was made, and fostering trust in the technology’s diagnostic capabilities.
By employing explainable AI techniques, researchers can now visually pinpoint the areas within carotid ultrasound images that most strongly influence the model’s assessment of vascular damage. This isn’t simply a matter of highlighting any region; the system identifies specific textural patterns, plaque formations, or wall thicknesses that correlate with the presence – or absence – of disease. These visual maps, often presented as heatmaps overlaid on the original ultrasound, reveal precisely what the algorithm is ‘seeing’ when it flags a potential issue. Consequently, clinicians gain a crucial window into the model’s decision-making process, enabling them to corroborate findings, identify potential errors, and ultimately, make more informed diagnoses.
The integration of explainable AI isn’t simply about achieving accurate diagnoses; it fundamentally shifts the dynamic between artificial intelligence and the clinician by providing a transparent rationale behind each assessment. Rather than functioning as an inscrutable ‘black box’, the model’s decision-making process is visualized, highlighting the precise image features – subtle indicators of vascular damage within carotid ultrasounds – that drove its conclusion. This visual confirmation allows medical professionals to independently verify the AI’s reasoning, fostering trust and enabling a more informed, collaborative approach to patient care. Consequently, clinicians can leverage the AI’s analytical power with greater confidence, knowing they possess the means to understand and validate its judgments, ultimately improving diagnostic accuracy and patient outcomes.
Analysis of vascular damage (VD) prevalence revealed notable demographic differences; at age 60, 42% of females exhibited low VD compared to 26% of males. Further investigation indicated a disproportionately higher rate of false negative diagnoses among younger males, suggesting the model’s current parameters may not be optimally calibrated for this specific subgroup. These findings highlight the importance of considering sex and age as potential confounding variables in automated assessments of vascular health and underscore the need for tailored algorithms or supplementary diagnostic procedures to improve accuracy across diverse patient populations.
The study’s success in identifying subtle vascular damage through machine learning echoes a fundamental truth: all systems, even biological ones, decay. The application of deep learning to carotid ultrasound isn’t merely a technological advancement; it’s a refined method of observing that inevitable decline. As Grace Hopper observed, “It’s easier to ask forgiveness than it is to get permission.” This sentiment applies to the proactive nature of early detection; the system doesn’t wait for catastrophic failure, but actively seeks the initial signals of deterioration. Refactoring, in this context, is the constant refinement of the diagnostic process, a dialogue with the past data to improve future predictions of cardiovascular risk. The non-invasive nature of the method allows for frequent ‘refactoring’-repeated assessment-to monitor the system’s evolution over time.
What’s Next?
Each commit in this line of inquiry is a record in the annals, and every version a chapter. This work, demonstrating the potential of deep learning to discern vascular damage from carotid ultrasound, doesn’t so much solve a problem as shift its boundaries. The initial promise of non-invasive risk assessment is compelling, yet the subtlety of ‘subtle damage’ invites scrutiny. The true test isn’t simply detection, but predictive power – whether these algorithmic glimpses into vascular health translate to meaningful interventions before irreversible cascade.
The current architecture, while demonstrably effective, is not immune to the entropy inherent in all systems. The dataset, however comprehensive, represents a specific cohort; generalization to diverse populations remains a crucial, and potentially fraught, exercise. Future iterations must grapple with the thorny issue of explainability – moving beyond ‘what’ is damaged to ‘why’, and offering clinicians not just a prediction, but a reasoned justification.
Delaying fixes – prioritizing model performance over robust, interpretable insights – is a tax on ambition. The field now faces a choice: pursue ever-increasing accuracy at the cost of understanding, or embrace a more holistic approach, acknowledging that even the most sophisticated algorithm is merely a tool, subject to the same limitations as the medium it inhabits.
Original article: https://arxiv.org/pdf/2602.17321.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- All Golden Ball Locations in Yakuza Kiwami 3 & Dark Ties
- All Itzaland Animal Locations in Infinity Nikki
- Elder Scrolls 6 Has to Overcome an RPG Problem That Bethesda Has Made With Recent Games
- Brent Oil Forecast
- Critics Say Five Nights at Freddy’s 2 Is a Clunker
- James Gandolfini’s Top 10 Tony Soprano Performances On The Sopranos
- Unlocking the Jaunty Bundle in Nightingale: What You Need to Know!
- Super Animal Royale: All Mole Transportation Network Locations Guide
- BREAKING: Paramount Counters Netflix With $108B Hostile Takeover Bid for Warner Bros. Discovery
- Gold Rate Forecast
2026-02-20 09:00