Author: Denis Avetisyan
Researchers have developed a forecasting model to anticipate peaks in pediatric respiratory infections, offering hospitals crucial time to prepare for increased demand.
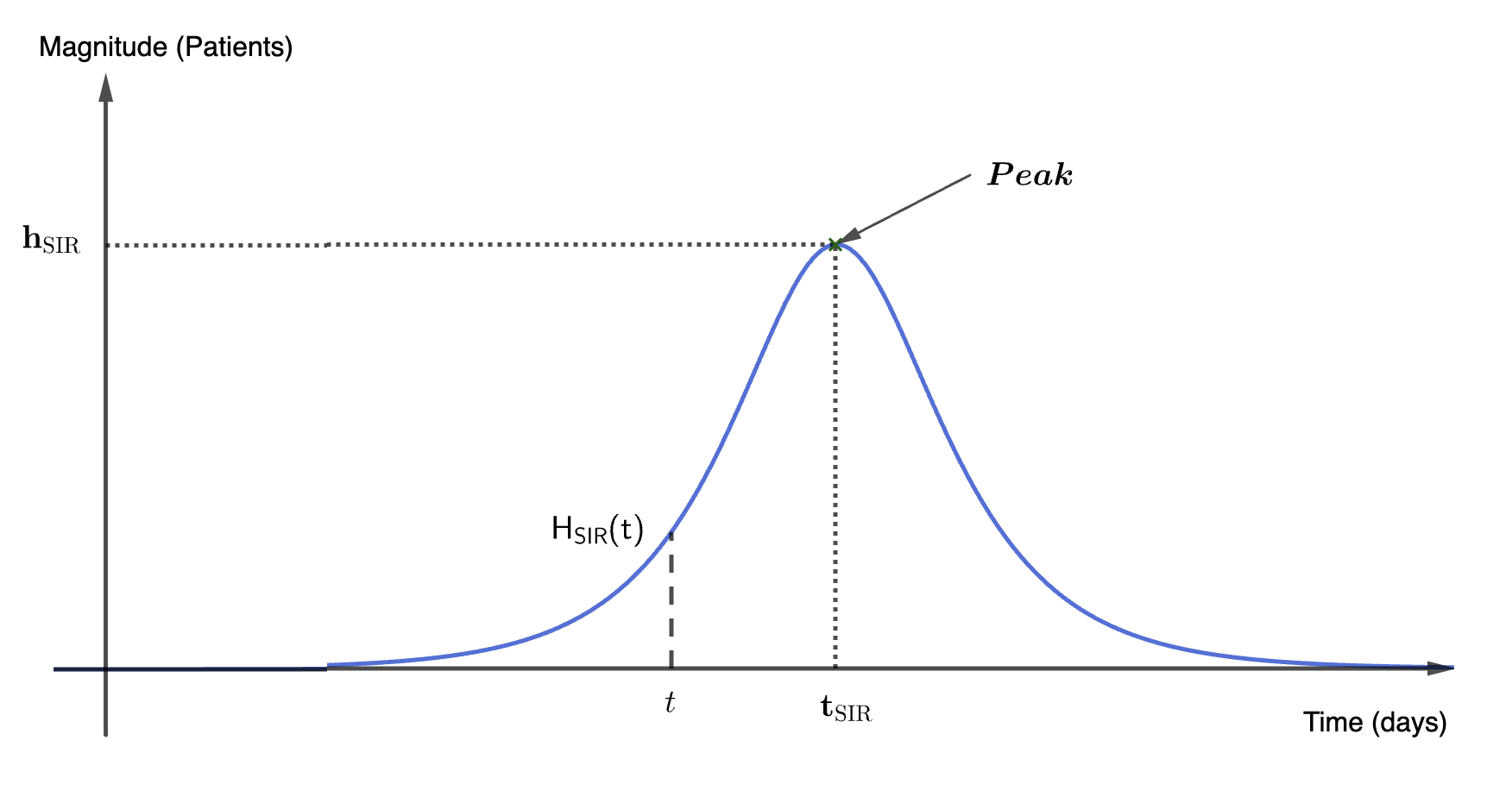
An alert-based framework combining historical trends and SIR dynamics accurately forecasts hospitalization peaks in Santiago, Chile.
Predicting seasonal surges in pediatric respiratory infections remains a persistent challenge for hospital resource allocation, despite established epidemiological patterns. This study, ‘Forecasting Seasonal Peaks of Pediatric Respiratory Infections Using an Alert-Based Model Combining SIR Dynamics and Historical Trends in Santiago, Chile’, introduces a novel forecasting framework that integrates historical trends with a mechanistic SIR model, activated by a derivative-based alert system. Results demonstrate the ability to anticipate hospitalization peaks approximately one month in advance and accurately predict magnitude two weeks prior, offering valuable lead time for preparedness. Could this approach be adapted to other regions and respiratory pathogens, improving global hospital resilience?
Emergent Patterns: Understanding Seasonal Respiratory Illness
Acute respiratory infections consistently pose a substantial health challenge for children in Santiago, Chile, with a clear tendency to surge during specific times of the year. These illnesses, encompassing common colds to more severe conditions like bronchiolitis and pneumonia, create predictable seasonal peaks largely coinciding with cooler months. This cyclical pattern isn’t random; it’s driven by factors such as increased indoor crowding, lower humidity facilitating viral survival, and potentially diminished immune function related to seasonal changes. The consistent recurrence of ARI cases places a considerable strain on pediatric healthcare resources, necessitating proactive planning and effective forecasting to ensure timely and adequate care for affected children. Understanding these entrenched seasonal dynamics is therefore critical for mitigating the impact of these prevalent infections within the city’s young population.
Conventional epidemiological forecasting methods frequently encounter difficulties when attempting to predict acute respiratory infection (ARI) outbreaks, particularly regarding both when cases will surge and how many hospitalizations will result. This predictive shortfall isn’t merely an academic problem; it directly impedes effective public health resource allocation. Hospitals and clinics struggle to proactively staff and supply themselves for anticipated peaks in patient volume, potentially leading to overwhelmed systems and compromised care. Furthermore, imprecise forecasts can result in wasted resources if predictions overestimate the outbreak’s severity, or, conversely, inadequate preparation if the outbreak exceeds expectations. The inherent complexity of ARI transmission, coupled with fluctuating environmental factors and population immunity levels, contributes to the persistent challenge of accurately anticipating these seasonal surges.
Accurate forecasting of acute respiratory infection (ARI) outbreaks demands a synthesis of epidemiological principles and real-world data. Traditional methods often fall short because they treat outbreaks as purely statistical phenomena, neglecting the underlying biological processes driving disease spread. This research demonstrates that a framework integrating mechanistic models – which account for factors like transmission rates and population immunity – with historical hospitalization data significantly improves predictive capabilities. Specifically, the developed system achieves reliable forecasts of peak outbreak dates more than one month in advance, offering a crucial advantage for proactive resource allocation and public health interventions. By bridging the gap between theoretical understanding and empirical observation, this approach represents a substantial step toward mitigating the impact of seasonal respiratory illnesses on pediatric populations.
A Framework Rooted in Mechanism and History
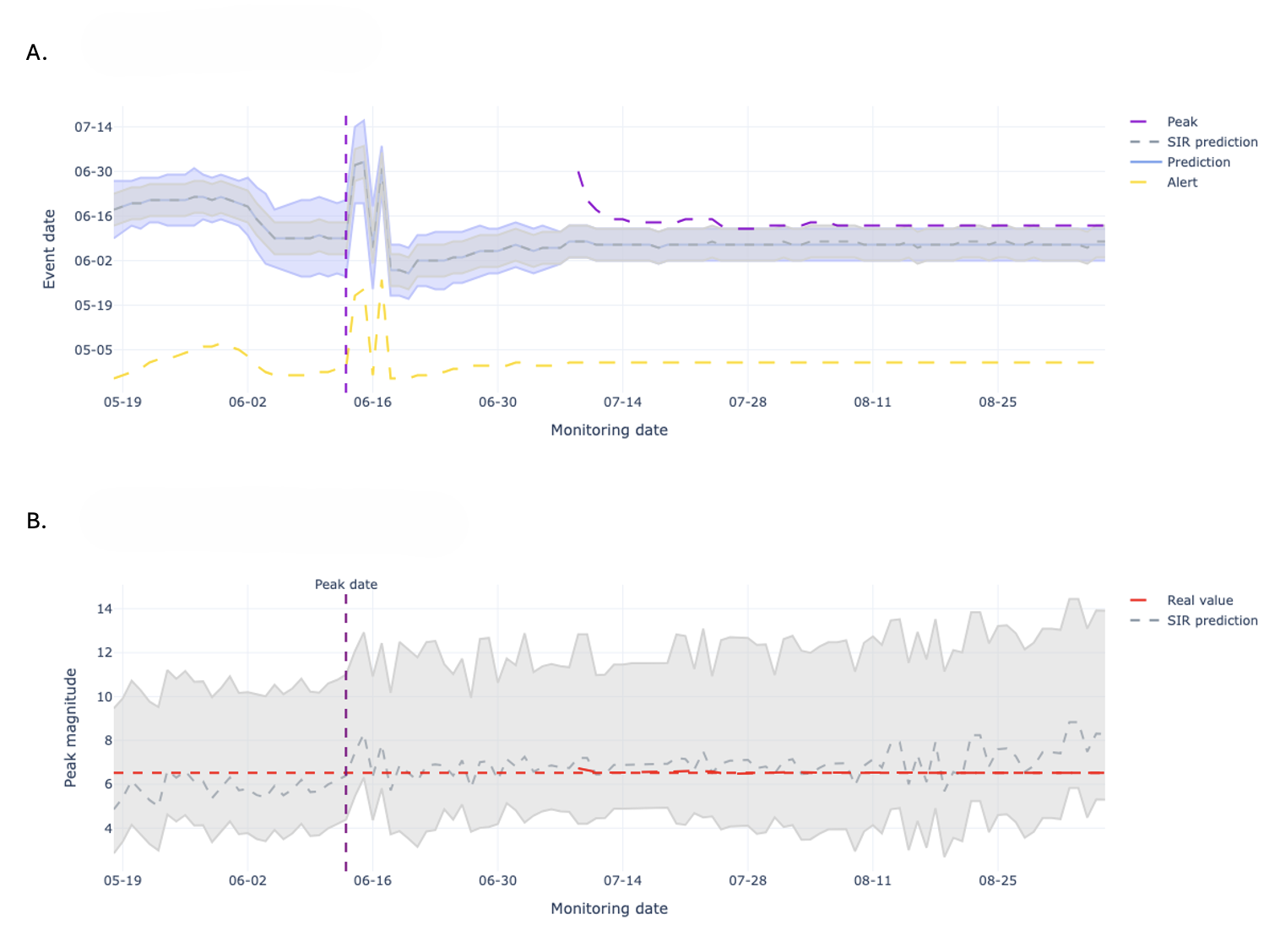
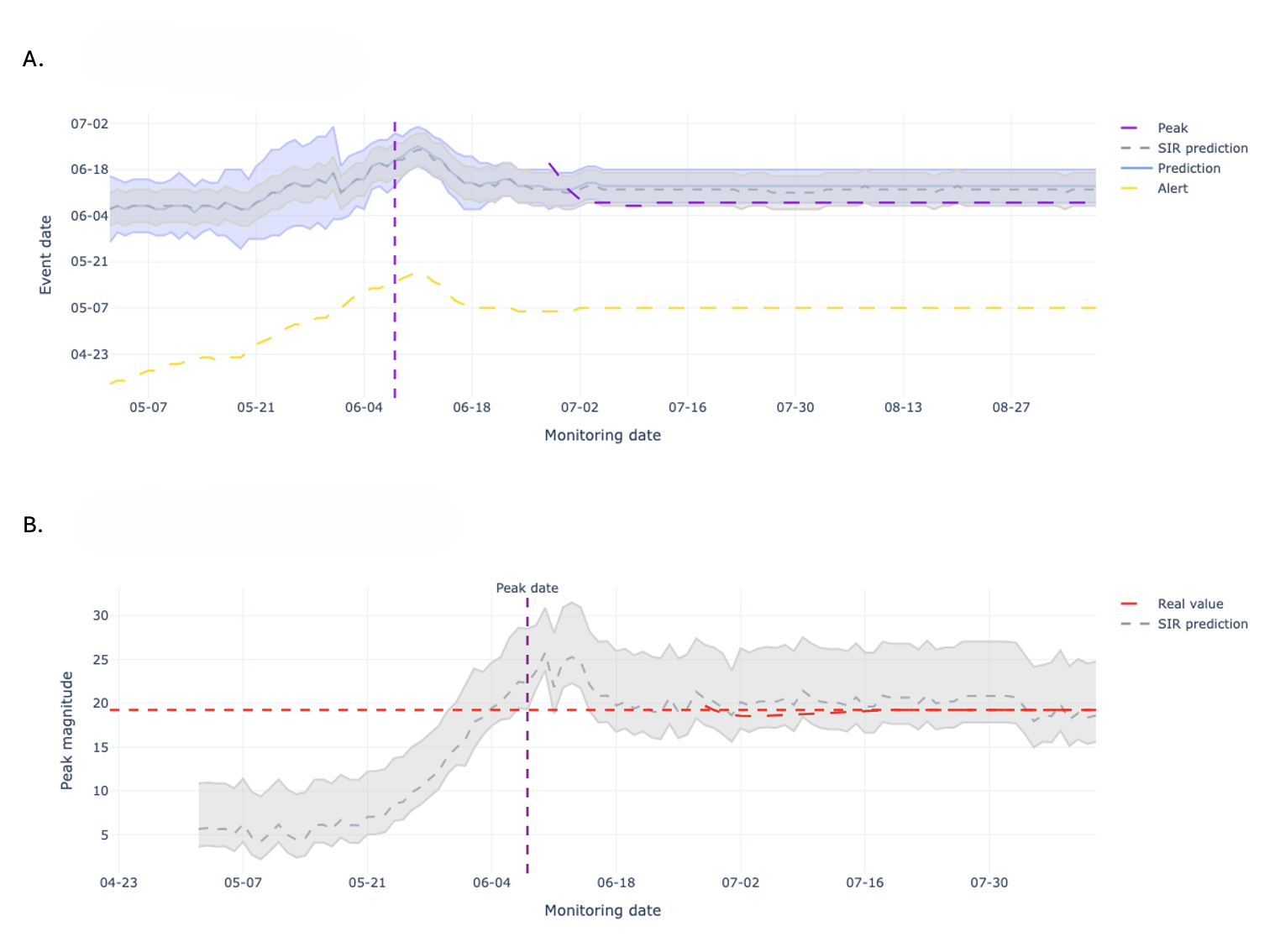
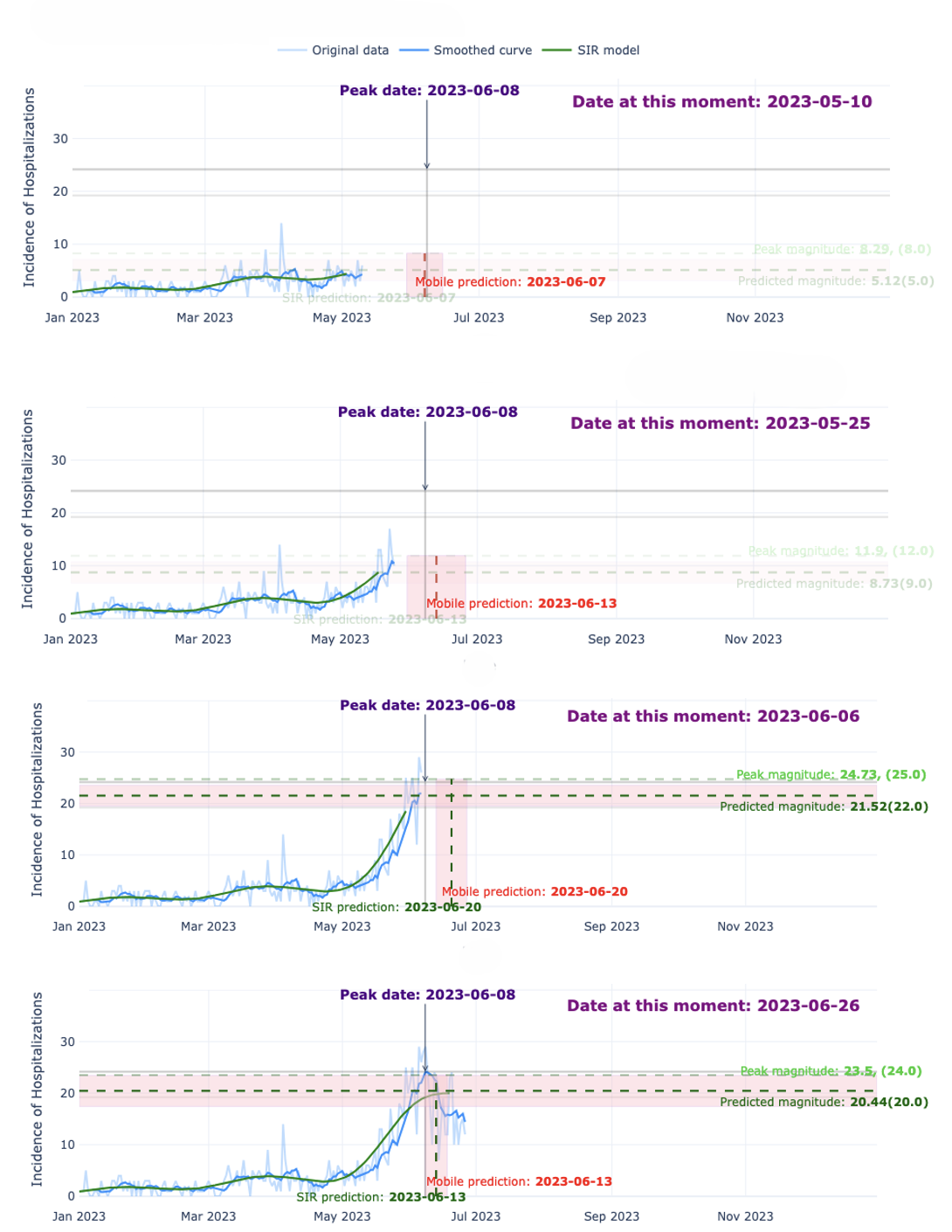
The Alert-Based Predictive Framework utilizes a Seasonal Susceptible-Infected-Recovered (SIR) model to simulate acute respiratory illness (ARI) transmission dynamics. This compartmental model divides the population into three states – susceptible, infected, and recovered – and uses differential equations to model the flow of individuals between these states. The seasonal component incorporates time-varying transmission rates, accounting for increased ARI incidence during typical respiratory virus seasons. Parameters within the SIR model, including transmission and recovery rates, are estimated using historical ARI surveillance data. The model’s output provides forecasts of infection prevalence and, consequently, expected hospitalization rates, forming the mechanistic basis for early-season predictions.
The Historical Mobile Prediction method functions as a complementary forecasting tool by leveraging patterns observed in prior years’ hospitalization data. This approach identifies annual peaks in respiratory illness-related hospitalizations and uses their timing and magnitude as predictors for the current season. Specifically, it calculates a moving average of past peak values to establish a baseline expectation for current hospitalization rates. This historical data is particularly valuable during the early stages of the respiratory illness season when the mechanistic Seasonal SIR model, reliant on real-time transmission data, has limited accuracy due to sparse initial observations. The method assumes that while the absolute magnitude of peaks may vary, the general temporal pattern of respiratory illness recurrence remains relatively consistent, allowing for proactive anticipation of future hospitalization trends.
The Alert-Based Predictive Framework employs a weighted combination of mechanistic and historical forecasting methods to optimize predictive accuracy throughout the Acute Respiratory Illness (ARI) season. Initially, greater weight is assigned to the Historical Mobile Prediction method, capitalizing on established patterns of hospitalization peaks when the Seasonal SIR model – the mechanistic component – has limited data for reliable short-term forecasts. As the season progresses and the SIR model accumulates data reflecting current transmission dynamics, its weighting increases. This dynamic adjustment ensures continuous predictive capability and enables peak magnitude forecasts to be accurate within a one-week timeframe, effectively mitigating the inherent uncertainty present in early-season predictions.
Refining the Signal: Data and Calibration
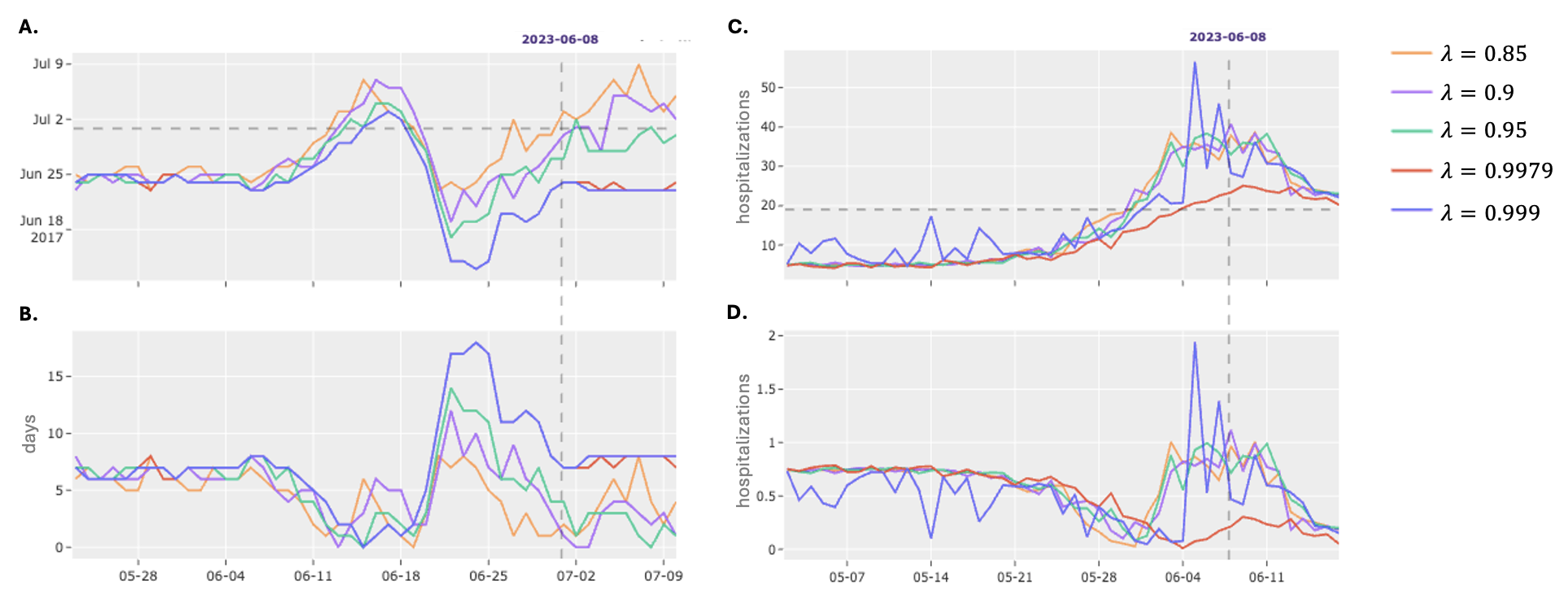
Data smoothing is a preprocessing step applied to daily hospitalization counts to mitigate the impact of reporting delays, weekend effects, and other random fluctuations. Specifically, we utilize a seven-day moving average to calculate smoothed hospitalization values, effectively reducing high-frequency noise in the time series. This smoothing process improves the performance of both the Seasonal SIR Model and the Historical Mobile Prediction by providing a more stable and representative input signal, leading to more accurate forecasts and reduced sensitivity to daily data anomalies. The smoothed data is then used as the primary input for model training and prediction, enhancing the reliability of peak date and peak magnitude estimations.
Model calibration is achieved through the minimization of a Loss Function, which serves as a quantitative measure of the discrepancy between model predictions and observed epidemiological data. This process involves adjusting model parameters to reduce the Loss Function value, thereby improving predictive accuracy. Specifically, hyperparameter fine-tuning is employed to identify the optimal combination of settings that satisfy pre-defined operational criteria: continuity, ensuring predictions do not exhibit abrupt shifts; alignment, verifying predicted trends match observed patterns; and stability, maintaining consistent performance across different datasets and time periods. The selection of an appropriate Loss Function – examples include Mean Squared Error or Root Mean Squared Error – is dependent on the specific characteristics of the data and the desired properties of the model output.
The Alert System functions as the primary mechanism for initiating predictive modeling cycles and disseminating actionable intelligence. It continuously monitors incoming data, triggering forecasts when pre-defined thresholds are met, thereby enabling proactive public health responses. Crucially, the system generates predictions for both the expected peak date of hospitalization rates and the anticipated peak magnitude – two key metrics informing intervention strategies. This dual forecasting capability allows public health officials to prepare resources and implement preventative measures with sufficient lead time, optimizing the effectiveness of interventions designed to mitigate the impact of disease outbreaks and ensure healthcare system capacity.
Beyond Prediction: Implications and Future Trajectories
An integrated forecasting framework has demonstrably improved the prediction of acute respiratory illness (ARI) hospitalization peaks in Santiago, Chile. This advancement enables public health officials to anticipate surges in patient volume with over a month’s lead time, facilitating proactive allocation of critical resources – including hospital beds, medical staff, and ventilators – to meet demand. The enhanced accuracy of these forecasts directly supports improved patient care by ensuring timely access to necessary treatment and minimizing the strain on the healthcare system during peak seasons. By moving beyond traditional statistical models, this approach offers a significant step towards more responsive and effective public health management of seasonal infectious diseases.
The forecasting framework, initially validated against acute respiratory infection (ARI) hospitalization data from Santiago, Chile, possesses a versatility extending beyond a single location or illness. Its core principles – integrating historical epidemiological data with meteorological factors and utilizing a weighted ensemble of forecasting models – are readily transferable to diverse geographical contexts and infectious diseases exhibiting pronounced seasonal trends. This adaptability stems from the framework’s reliance on fundamental epidemiological patterns rather than disease-specific characteristics, suggesting potential applications in predicting seasonal outbreaks of influenza, norovirus, or even vector-borne diseases in regions with comparable climatic conditions and data availability. Consequently, public health organizations facing predictable seasonal surges in infectious disease incidence could leverage this adaptable methodology to proactively allocate resources, optimize healthcare preparedness, and ultimately mitigate the impact on vulnerable populations.
Ongoing research seeks to bolster the predictive capabilities of this framework by integrating continuously updated, real-time data streams – encompassing sources like emergency room visits, social media trends related to symptom reporting, and even environmental factors – directly into the modeling process. Simultaneously, efforts are concentrated on refining the weighting algorithm that prioritizes various data inputs, aiming to optimize its responsiveness to subtle shifts in disease dynamics. This iterative process of data integration and algorithmic refinement promises not only heightened forecast accuracy, but also a more nuanced understanding of acute respiratory infection transmission, ultimately enabling public health officials to implement targeted interventions with greater precision and effectiveness.
The study demonstrates how a synthesis of historical observation and mechanistic modeling-specifically, the SIR model-can generate predictive alerts for pediatric respiratory infections. This approach mirrors the ancient wisdom of Epicurus, who observed that “the greatest pleasure of life is wisdom.” The forecasting framework doesn’t attempt to control epidemic peaks, an illusion given the complex interplay of factors, but rather to influence preparedness through anticipatory alerts. Each alert, a local change within the hospital system, resonates through the network of resource allocation, potentially producing colossal effects in patient care and optimized hospital function. The model’s predictive capacity isn’t about dominating the system, but understanding its inherent tendencies, a principle deeply aligned with embracing natural processes.
Beyond the Peak
The pursuit of epidemic peak prediction, as exemplified by this work, often feels like attempting to steer a river. One can anticipate the flood, perhaps even mitigate some damage with strategic diversions, but the river ultimately flows where the terrain dictates. This alert-based framework, blending historical observation with mechanistic modeling, represents a sensible approach – not toward control, but toward informed responsiveness. The true utility lies not in precise forecasting, an inherently limited goal in complex systems, but in providing hospitals with actionable lead time – space to adapt, rather than to rigidly prepare.
Future iterations should resist the temptation to layer on complexity in pursuit of improved accuracy. Instead, attention might focus on quantifying the model’s inherent uncertainty, and crucially, on understanding how the system responds to the alerts themselves. Does advance warning change behavior – resource allocation, public health messaging – and if so, how does that feedback alter the very peaks the model seeks to predict? Acknowledging the system’s self-modifying nature is paramount.
Ultimately, this work reinforces a broader principle: in complex systems, it’s better to encourage local rules than build hierarchy. A hospital prepared to be flexible, to adjust to incoming signals, will likely fare better than one rigidly adhering to a pre-ordained plan. System outcomes are unpredictable but resilient, and true progress lies in fostering that resilience, rather than chasing the illusion of prediction.
Original article: https://arxiv.org/pdf/2601.09821.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- All Golden Ball Locations in Yakuza Kiwami 3 & Dark Ties
- The MCU’s Mandarin Twist, Explained
- These are the 25 best PlayStation 5 games
- Movie Games responds to DDS creator’s claims with $1.2M fine, saying they aren’t valid
- Scream 7 Will Officially Bring Back 5 Major Actors from the First Movie
- Server and login issues in Escape from Tarkov (EfT). Error 213, 418 or “there is no game with name eft” are common. Developers are working on the fix
- SHIB PREDICTION. SHIB cryptocurrency
- A Knight Of The Seven Kingdoms Season 1 Finale Song: ‘Sixteen Tons’ Explained
- Gold Rate Forecast
- All Songs in Helluva Boss Season 2 Soundtrack Listed
2026-01-18 19:54