Author: Denis Avetisyan
A new approach combines the strengths of neural networks and traditional epidemiological models to overcome the challenges of noisy, incomplete data and improve prediction accuracy.
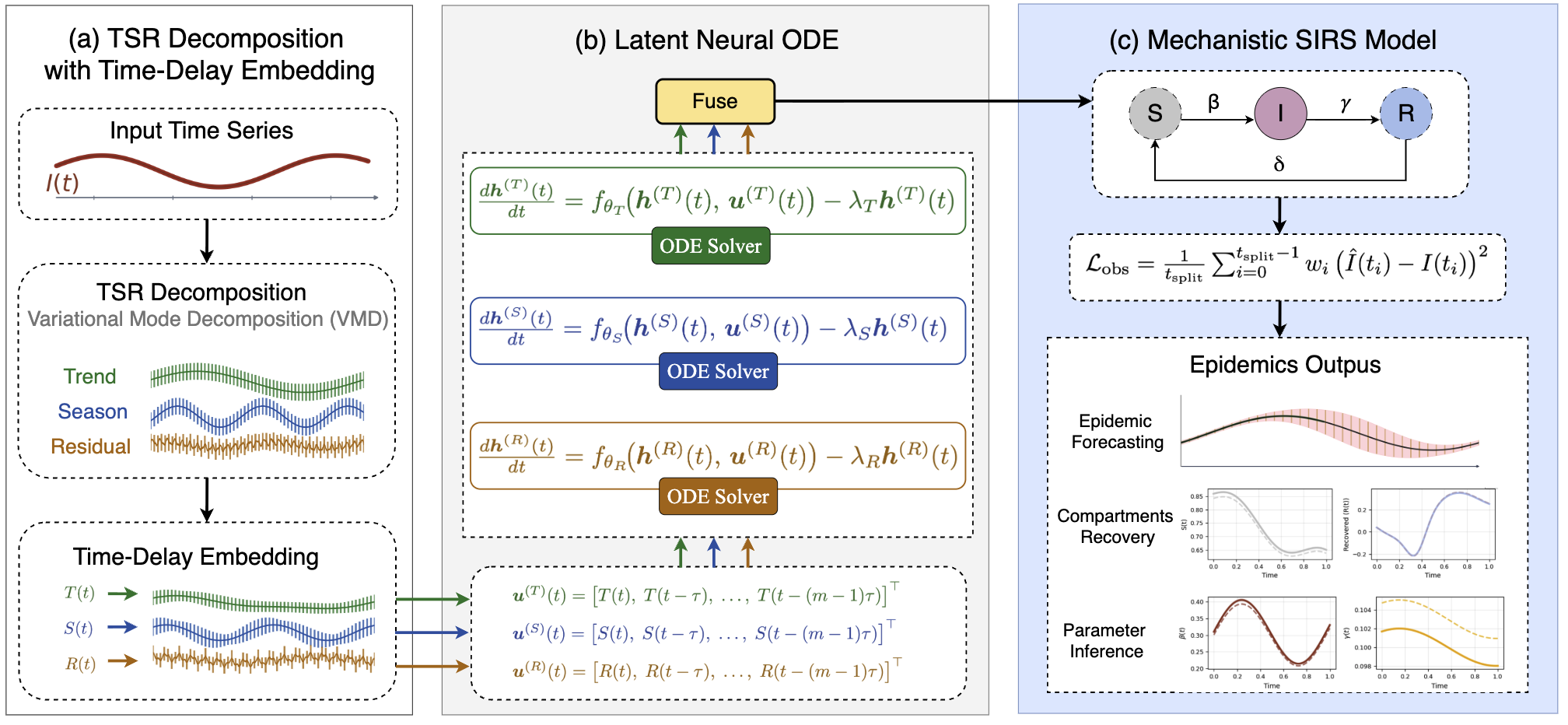
This review introduces EpiNode, a hybrid neural-mechanistic model leveraging multi-scale dynamics and time series decomposition for robust epidemiological forecasting under partial observability.
Accurate epidemiological forecasting remains a persistent challenge despite advances in time-series modeling and mechanistic approaches. This paper, ‘How (Not) to Hybridize Neural and Mechanistic Models for Epidemiological Forecasting’, addresses the limitations of naively combining these paradigms by demonstrating that robust performance requires explicitly modeling the multi-scale, non-stationary dynamics inherent in disease transmission. We introduce EpiNode, a hybrid model that decomposes observed infections into interpretable components-trend, seasonality, and residuals-to drive a controlled neural ODE coupled with an epidemiological framework. By jointly forecasting and inferring time-varying parameters, can this approach unlock more reliable, long-horizon predictions and improve our understanding of complex epidemic behavior?
Decoding the Chaos: The Limits of Static Epidemic Models
Conventional epidemic modeling frequently relies on the simplification of static conditions, a practice that often clashes with the dynamic reality of disease transmission. These models typically presume consistent parameters – such as transmission rates and population susceptibility – over time, neglecting the significant influence of fluctuating factors. Real-world epidemics are rarely stable; they are shaped by changes in human behavior – like mask-wearing or social distancing – seasonal variations that affect viral survival and transmission, and evolving viral characteristics, such as increased infectivity or immune evasion. Consequently, the rigid assumptions inherent in static models can lead to inaccurate predictions and a limited understanding of how epidemics truly unfold, hindering effective public health interventions. The failure to account for these dynamic elements represents a critical limitation in current epidemiological forecasting.
Epidemic time series are rarely stable; their inherent non-stationarity presents a significant hurdle to accurate forecasting. Unlike predictable patterns, disease transmission is often shaped by fluctuating environmental factors, most notably seasonality – consider influenza’s winter peaks or mosquito-borne illnesses linked to warmer months. However, seasonality is just one facet; human behavior, including responses to public health interventions like masking or social distancing, dynamically alters transmission rates. These behavioral shifts, coupled with evolving viral characteristics or changes in population immunity, introduce complexities that invalidate assumptions of constant parameters in traditional models. Consequently, forecasts built on stationary assumptions can quickly diverge from reality, highlighting the need for adaptive modeling approaches capable of capturing these ever-changing dynamics and acknowledging that past trends are not necessarily indicative of future outbreaks.
Epidemiological modeling is fundamentally challenged by the reality of partial observability – the impossibility of comprehensively tracking every infection within a population. Because a significant proportion of cases often go undetected, either due to asymptomatic presentation or limited testing capacity, standard models rely on estimates derived from observed cases to infer the true extent of the outbreak. This introduces substantial uncertainty into predictions of disease spread, as the unobserved portion of the epidemic can significantly influence its trajectory. Researchers are actively developing techniques – including sophisticated statistical inference and nowcasting methods – to account for this hidden mass of infections and refine forecasts, but inherent limitations remain. Effectively addressing partial observability is crucial not only for accurate prediction, but also for evaluating the impact of interventions and informing public health strategies.
Beyond Simplification: Modeling the Evolving Epidemic
The SEIRS model is a compartmental model used in epidemiology that builds upon the foundational SIRS (Susceptible-Infected-Recovered-Susceptible) framework by adding an ‘Exposed’ compartment. This extension acknowledges that many diseases have an incubation period – a time between infection and becoming infectious – which is not captured in the basic SIRS model. Individuals transition from Susceptible to Exposed after infection, remaining non-infectious during this latent period. Following the exposed stage, individuals then become Infectious, capable of transmitting the disease, before eventually Recovering and returning to the Susceptible compartment, potentially losing immunity. The inclusion of the Exposed compartment provides a more realistic representation of disease dynamics, particularly for pathogens with a significant incubation period, and allows for a more accurate estimation of key epidemiological parameters like R_0.
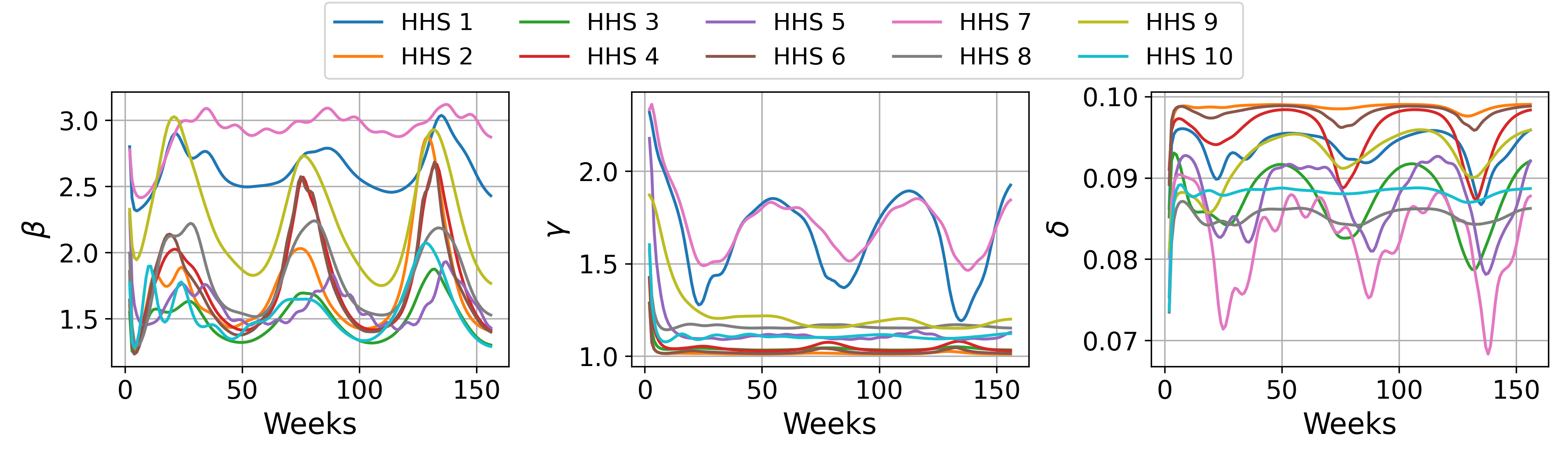
Epidemic models traditionally employ fixed parameters to define transmission rates; however, these rates are not static in real-world scenarios. Incorporating time-varying parameters allows models to reflect the impact of interventions – such as mask mandates, vaccination campaigns, or social distancing measures – which directly alter the rate of infection \beta(t). Similarly, behavioral changes in the population, like increased handwashing or reduced social interaction, also influence transmission dynamics and can be represented by adjusting parameters over time. This dynamic modeling approach improves predictive accuracy by acknowledging that factors affecting disease spread are not constant, but evolve throughout the course of an epidemic.
Seasonal forcing in epidemiological modeling addresses the non-stationary characteristic of many infectious diseases by incorporating periodic fluctuations in transmission rates. This technique acknowledges that disease incidence often exhibits predictable annual or other periodic patterns due to factors like temperature, humidity, or human behavior changes-such as school terms or holiday travel. Mathematically, seasonal forcing is typically implemented by modulating parameters like the transmission rate β with a sinusoidal function, allowing the model to simulate recurring outbreaks or declines in incidence. The general form often used is \beta(t) = \beta_0 + A \cos(2\pi t / T) , where \beta_0 is the baseline transmission rate, A represents the amplitude of the seasonal variation, and T is the period of the seasonal cycle, usually one year. Accurately representing this seasonality improves model fit and predictive capability for diseases like influenza, respiratory syncytial virus (RSV), and norovirus.
EpiNode: A Hybrid Approach to Forecasting
EpiNode is a forecasting framework designed to combine the advantages of both mechanistic and data-driven modeling techniques. It leverages Susceptible-Infected-Recovered (SIRS) models, which incorporate established epidemiological principles, with Neural Ordinary Differential Equations (Neural ODEs) – a class of deep learning models capable of learning complex temporal dynamics from data. This hybrid approach allows EpiNode to benefit from the interpretability and theoretical grounding of SIRS models while simultaneously exploiting the flexibility and learning capacity of Neural ODEs to capture nuanced patterns present in observed epidemic data. The framework effectively bridges the gap between traditional compartmental models and fully data-driven methods, aiming to provide more accurate and robust epidemic predictions.
EpiNode utilizes Time-Delay Embedding (TDE) to address the challenge of reconstructing epidemic dynamics from limited observational data. TDE creates a higher-dimensional state space by incorporating past values of the observed epidemic time series as additional features. This allows the model to capture the temporal dependencies inherent in disease transmission, effectively estimating the underlying dynamics even with sparse or noisy data. By reconstructing these dynamics, EpiNode improves the accuracy of short- and long-term epidemic forecasts compared to models that rely solely on current observations or simplistic time series extrapolations. The technique effectively expands the information available to the forecasting model without requiring explicit knowledge of the underlying transmission mechanisms.
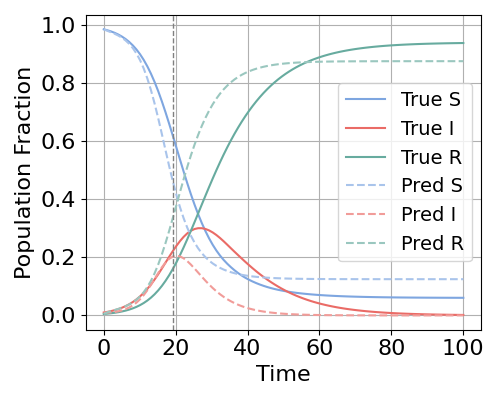
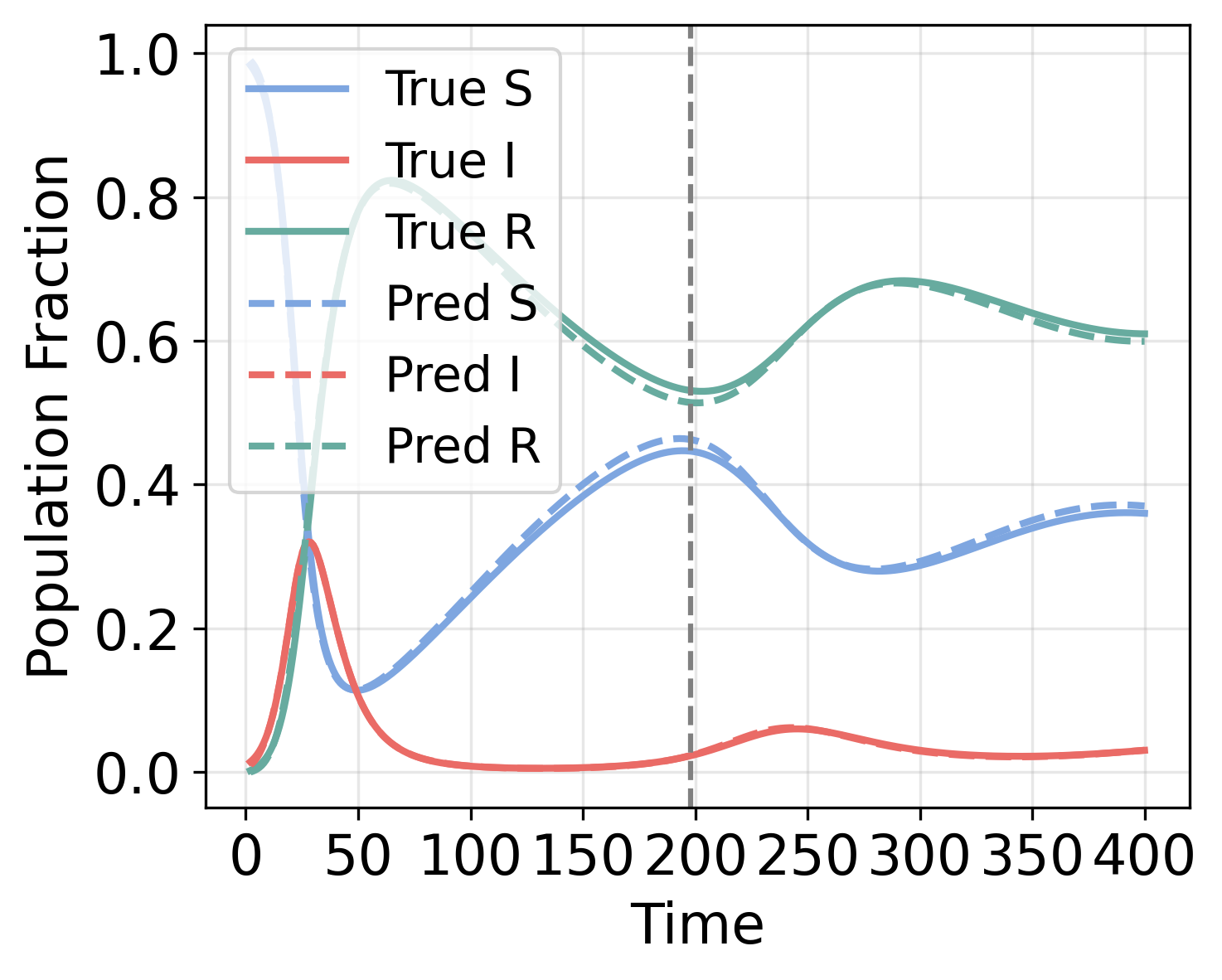
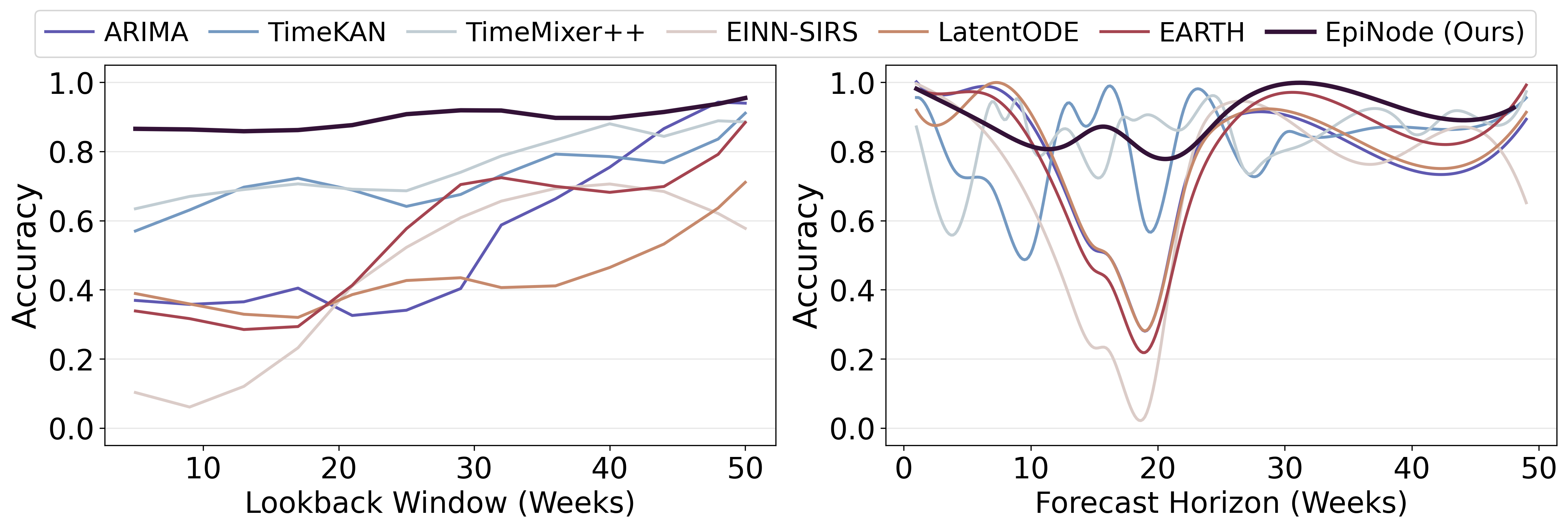
EpiNode consistently achieved state-of-the-art performance in forecasting tasks, as evidenced by the lowest Root Mean Squared Error (RMSE) values across all tested datasets, including both synthetically generated and real-world epidemiological data. Comparative analysis demonstrated superior performance relative to established time-series forecasting models such as ARIMA, recurrent neural networks (RNNs), and more recent architectures including TimeKAN, TimeMixer++, EINN, and other ODE-based modeling approaches. This consistent outperformance indicates EpiNode’s effectiveness in capturing complex epidemiological dynamics and producing accurate predictions.
Bounded parameterization within the EpiNode framework enforces biologically plausible ranges for model parameters, contributing to both stability and interpretability. Specifically, parameters representing rates of infection, recovery, and disease-induced death are constrained based on established epidemiological principles and observed data distributions. This restriction prevents the model from exploring unrealistic parameter spaces that could lead to unstable predictions or nonsensical results. By limiting parameter values to meaningful intervals, EpiNode enhances the reliability of forecasts and facilitates a clearer understanding of the underlying epidemic dynamics, allowing for more informed public health interventions and scenario planning.
Decoding the Future: Implications for Public Health and Beyond
Precisely determining when and how intensely an epidemic will peak is paramount for effective public health responses. Accurate forecasts of peak timing allow authorities to proactively allocate critical resources – hospital beds, ventilators, personal protective equipment, and staffing – to meet anticipated demand, preventing healthcare systems from becoming overwhelmed. Simultaneously, forecasting peak magnitude informs the intensity of non-pharmaceutical interventions, such as social distancing measures or school closures, ensuring they are proportionate to the projected burden and minimizing societal disruption. Without reliable predictions of these key parameters, interventions risk being either insufficient to control the outbreak or unnecessarily restrictive, leading to economic hardship and public fatigue. Consequently, advancements in forecasting capabilities represent a vital tool for mitigating the impact of future epidemics and safeguarding public health.
EpiNode distinguishes itself through a marked reduction in forecasting bias concerning both the timing and magnitude of epidemic peaks, a critical advantage demonstrated particularly with synthetic datasets designed to rigorously test predictive capabilities. Evaluations reveal EpiNode consistently outperforms alternative models in accurately pinpointing when an outbreak will reach its highest point and how large that peak will be. This improved precision isn’t simply a matter of statistical refinement; it stems from the model’s capacity to integrate mechanistic understanding of disease spread with data-driven learning. Consequently, EpiNode offers a more reliable basis for preemptive public health interventions, allowing for optimized resource allocation and targeted control strategies when facing emerging infectious diseases.
EpiNode leverages the power of Latent Ordinary Differential Equations (Latent ODEs) to move beyond simply tracking observable cases and instead model the hidden, underlying states of a disease within a population. This approach acknowledges that infection isn’t an instantaneous event, but a process unfolding across varying levels of susceptibility and exposure – aspects traditionally obscured in standard epidemiological models. By inferring these latent states, such as the proportion of individuals pre-symptomatically infected or the level of ambient viral load, EpiNode gains a more nuanced understanding of transmission dynamics. This capability allows for a more accurate depiction of how a disease progresses through a population, revealing critical periods for intervention and offering insights into the effectiveness of different control strategies. The resulting framework doesn’t merely predict what will happen, but provides a mechanistic basis for understanding why, ultimately enhancing preparedness for future outbreaks.
EpiNode establishes a powerful framework for epidemic preparedness by integrating the strengths of both mechanistic modeling and data-driven techniques. Traditional mechanistic models, built upon established epidemiological principles, often struggle to adapt to the nuances of real-world outbreaks due to incomplete knowledge or simplifying assumptions. Conversely, purely data-driven approaches, while flexible, can lack the interpretability and predictive power needed for novel scenarios. EpiNode bridges this gap, leveraging Latent Ordinary Differential Equations to capture underlying disease dynamics while simultaneously refining these dynamics with observed data. This hybrid approach not only improves forecasting accuracy – particularly for peak timing and magnitude – but also provides valuable insights into the unseen states governing disease transmission. By accommodating both prior knowledge and emerging evidence, EpiNode offers a robust and adaptable system for anticipating and mitigating the challenges posed by future epidemics, moving beyond reactive responses toward proactive epidemic management.
The pursuit within EpiNode-a system designed to untangle complex epidemiological forecasting-mirrors a fundamental tenet of exploration. Ken Thompson famously stated, “Debugging is like being the detective in a crime movie where you are also the murderer.” This sentiment applies directly to the challenges addressed by the model. EpiNode doesn’t simply accept the data; it actively dissects the multi-scale dynamics, acknowledging the inherent ‘murkiness’ of partial observability and non-stationarity. The process necessitates a willingness to challenge assumptions-to ‘break’ the initial understanding of the data-in order to construct a more robust and accurate forecasting system, much like tracing the source of a complex error.
Beyond the Forecast
EpiNode, in its attempt to reconcile the rigidity of mechanistic models with the flexibility of neural networks, doesn’t so much solve the problem of epidemiological forecasting as it systematically isolates new points of failure. The model’s success hinges on decomposing time series, a process that, while effective, merely shifts the burden of proof onto the decomposition algorithm itself. One begins to suspect that all forecasting is ultimately an exercise in carefully constructing increasingly elaborate methods for postponing inevitable error.
The explicit modeling of multi-scale dynamics is a step, certainly, but it also highlights a deeper question: are we truly seeking to understand epidemics, or merely to predict their trajectories? The focus on partial observability, while pragmatic, feels like an admission that a complete understanding remains elusive – a tacit acknowledgement that the system will always resist full reconstruction. Future work shouldn’t simply refine EpiNode’s parameters, but interrogate the very premise of hybrid modeling – whether stitching together different levels of abstraction genuinely yields insight, or simply creates a more complex black box.
The true challenge lies not in minimizing forecast error, but in designing models that fail informatively. A model that clearly articulates the limits of its knowledge, that identifies precisely where and why its predictions break down, is ultimately more valuable than one that offers spurious accuracy. It is in the careful study of these failures that genuine understanding – and perhaps, eventual control – may reside.
Original article: https://arxiv.org/pdf/2602.06323.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- All Golden Ball Locations in Yakuza Kiwami 3 & Dark Ties
- The MCU’s Mandarin Twist, Explained
- Movie Games responds to DDS creator’s claims with $1.2M fine, saying they aren’t valid
- These are the 25 best PlayStation 5 games
- Scream 7 Will Officially Bring Back 5 Major Actors from the First Movie
- SHIB PREDICTION. SHIB cryptocurrency
- Server and login issues in Escape from Tarkov (EfT). Error 213, 418 or “there is no game with name eft” are common. Developers are working on the fix
- A Knight Of The Seven Kingdoms Season 1 Finale Song: ‘Sixteen Tons’ Explained
- Rob Reiner’s Son Officially Charged With First Degree Murder
- Every Death In The Night Agent Season 3 Explained
2026-02-09 13:50