Author: Denis Avetisyan
A new deep learning framework dramatically accelerates the prediction of blood flow dynamics within brain aneurysms, offering a faster path to risk assessment.
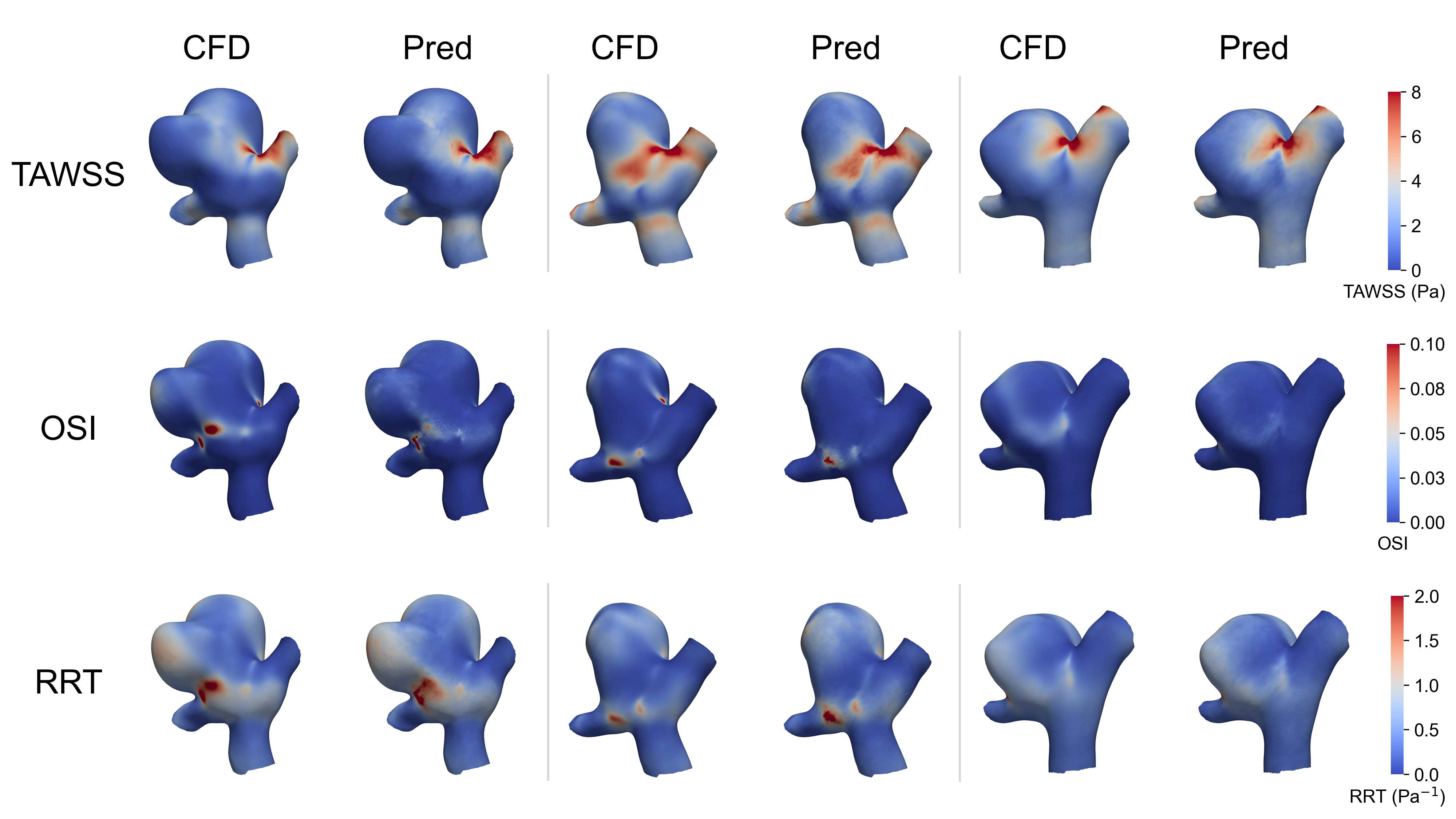
This work introduces a real-time hemodynamics surrogation method using graph neural networks to efficiently predict wall shear stress in non-idealized intracranial aneurysms.
Despite established links between fluid biomechanics and intracranial aneurysm (IA) progression, clinical translation of computationally derived risk indicators remains limited by the expertise and time required for traditional Computational Fluid Dynamics (CFD) simulations. This work, ‘RHSIA: Real-time Hemodynamics Surrogation for Non-idealized Intracranial Aneurysms’, introduces a deep learning framework leveraging Graph Transformer networks to accurately predict transient wall shear stress (WSS) directly from IA surface meshes, achieving high fidelity with a Structural Similarity Index of up to 0.981. By incorporating temporal information and utilizing data augmentation with low-cost steady-state CFD, the model offers a computationally efficient surrogate for full CFD analysis, even with limited pulsatile data. Could this approach unlock real-time, personalized risk assessment for patients with IAs and broaden the application of surrogate modeling in cardiovascular biomechanics?
The Inevitable Failure of Prediction
Intracranial aneurysms represent a serious threat to patient health, with rupture often resulting in devastating consequences such as subarachnoid hemorrhage, stroke, and even death. Despite advancements in medical imaging, the ability to reliably predict which aneurysms will rupture remains a substantial clinical challenge. The inherent difficulty lies in the complex interplay of factors influencing aneurysm stability; many remain asymptomatic and stable throughout a patient’s life, while others exhibit unpredictable growth and a heightened risk of rupture. This unpredictability necessitates ongoing research into more accurate risk stratification methods, as current approaches often struggle to differentiate between truly high-risk lesions and those that pose minimal immediate danger, leaving clinicians facing a critical diagnostic dilemma.
Currently, the evaluation of intracranial aneurysms heavily depends on visualizing their shape and characteristics through medical imaging techniques such as Digital Subtraction Angiography, Computed Tomography Angiography, and Magnetic Resonance Angiography. These methods excel at identifying the aneurysm’s size, location, and general morphology – features clinicians traditionally use to gauge rupture risk. However, these morphological assessments often prove insufficient in predicting which aneurysms will actually rupture. Subtle changes in wall stress, hemodynamics, or material properties – crucial indicators of instability – are not readily apparent through standard imaging. This limitation stems from the fact that an aneurysm’s outward appearance doesn’t always reflect its internal biomechanical vulnerabilities, leading to both false positives – unnecessary interventions on stable aneurysms – and, more critically, false negatives where high-risk lesions are overlooked.
Existing clinical scoring systems, like PHASES and ELAPSS, represent an attempt to categorize intracranial aneurysm risk, yet their utility is hampered by a reliance on broad morphological characteristics and a subsequent lack of precision. While these systems can broadly stratify aneurysms into risk categories, they frequently fail to distinguish between lesions that will ultimately rupture and those that will remain stable, leading to both unnecessary interventions and potentially fatal oversight. This limitation stems from the complex interplay of factors influencing aneurysm stability – hemodynamics, wall properties, and patient-specific predispositions – which are not fully captured by current scoring methods. Consequently, research is actively focused on developing more refined risk assessment tools that incorporate advanced imaging techniques, computational modeling, and potentially even biomarkers, to achieve a more granular understanding of aneurysm vulnerability and ultimately improve patient outcomes.
The Illusion of Control Through Hemodynamics
Hemodynamic parameters, most notably Wall Shear Stress (WSS), are now understood to be primary contributors to both aneurysm formation and the risk of rupture. Elevated WSS, exceeding physiological limits, induces endothelial dysfunction and remodeling of the vessel wall, potentially leading to weakening and expansion. Conversely, low or oscillatory WSS promotes endothelial cell detachment and contributes to the development of intimal hyperplasia. The magnitude and pattern of WSS distribution are not uniform within an aneurysm; regions experiencing consistently high or fluctuating stress are statistically more prone to rupture than areas with stable, moderate stress levels. These effects are quantified by metrics like \tau_w = \mu \frac{du}{dy}, where \tau_w represents WSS, μ is the dynamic viscosity of blood, and \frac{du}{dy} is the velocity gradient perpendicular to the vessel wall.
Detailed quantification of hemodynamic parameters relies heavily on Computational Fluid Dynamics (CFD), a technique involving the numerical solution of governing equations for fluid flow. This process requires the creation of a three-dimensional geometric model of the aneurysm and surrounding vasculature, followed by discretization of the model into a mesh of elements. Solving the equations for each element, accounting for blood viscosity and density, necessitates significant computational resources, including high-performance computing infrastructure. Furthermore, accurate CFD simulations demand substantial processing time – often several days or weeks – to achieve convergence and reliable results, particularly for complex aneurysm geometries or time-dependent flow conditions. The computational cost scales with model complexity, mesh resolution, and the duration of simulated blood flow.
4D Flow MRI provides direct visualization of blood velocity within aneurysms, offering a non-invasive method for hemodynamic assessment. However, the technique is constrained by inherent limitations in spatial resolution, typically on the order of 1-3 mm3, which can obscure fine-scale flow structures and accurate wall shear stress calculation. Furthermore, 4D Flow MRI is susceptible to artifacts arising from patient motion, magnetic field inhomogeneities, and aliasing, especially in complex vascular geometries. These artifacts can introduce inaccuracies in velocity measurements, particularly at vessel bifurcations or in regions of turbulent flow, thereby reducing the reliability of the derived hemodynamic parameters and potentially impacting the assessment of aneurysm stability. The signal-to-noise ratio can also be a limiting factor, necessitating longer scan times or compromising resolution to achieve acceptable image quality.
Time-Averaged Wall Shear Stress (TAWSS) and Relative Residence Time (RRT) provide more detailed assessments of wall loading than instantaneous measurements. TAWSS, expressed in Pascals, represents the mean shear stress experienced by the endothelial cells over a cardiac cycle and is considered a primary indicator of endothelial cell behavior and remodeling. RRT quantifies the duration for which fluid remains in close proximity to the vessel wall, normalized by the cardiac cycle length; lower RRT values correlate with areas of increased endothelial activation and potential rupture risk. Combining TAWSS and RRT allows for a more comprehensive understanding of the hemodynamic environment, differentiating between regions of sustained stress and those experiencing fluctuating or low-magnitude forces, which are both implicated in aneurysm pathogenesis. These refined measures, calculated from velocity fields obtained via 4D Flow MRI or CFD, enable identification of high-risk zones not readily apparent through simple peak stress analysis.
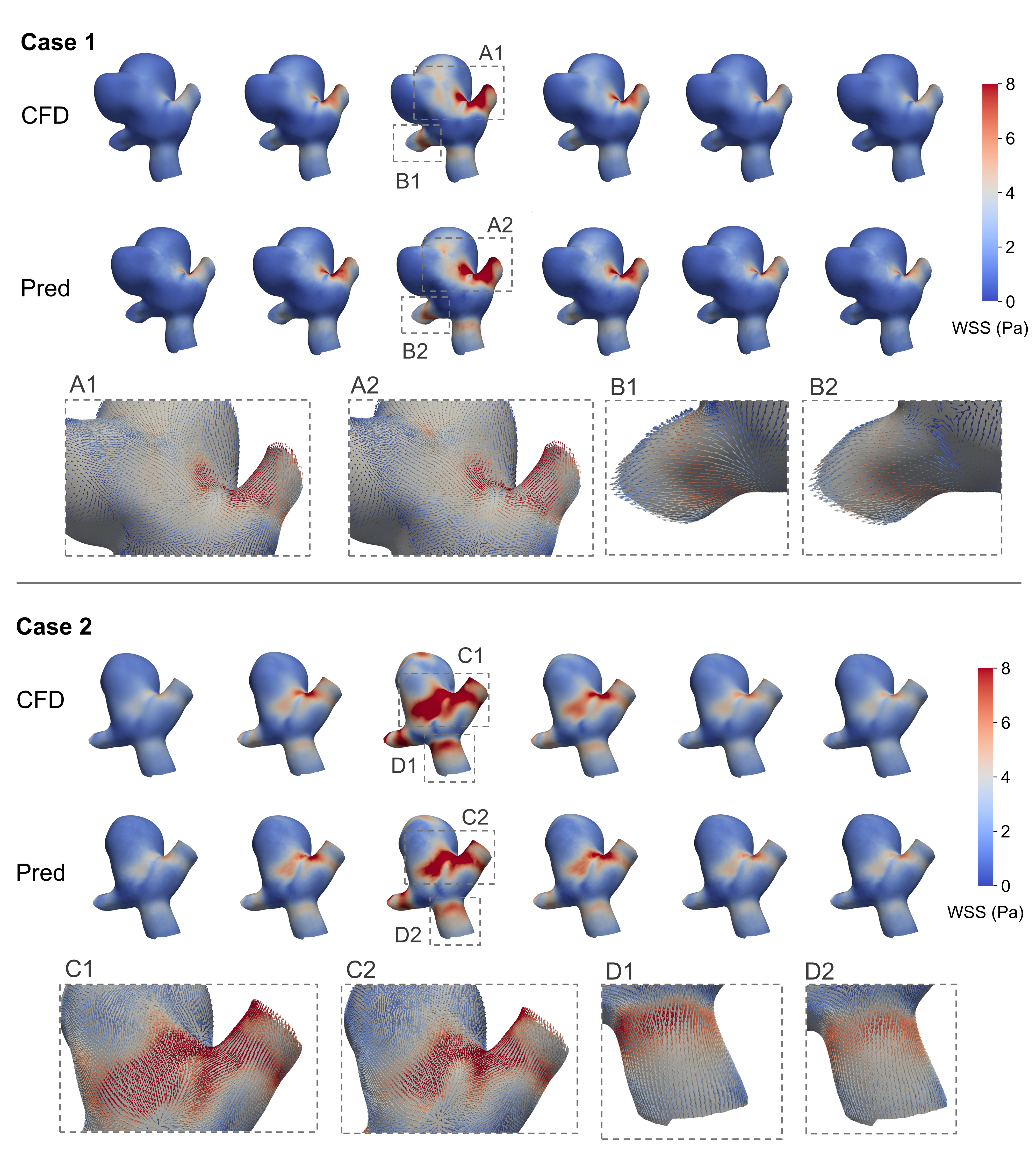
The Mirage of Speed: Deep Learning as a Temporary Stay
Computational Fluid Dynamics (CFD) provides detailed insight into hemodynamic parameters within aneurysms, but is computationally expensive and time-consuming. Deep learning techniques offer a potential solution by approximating CFD simulations, enabling significantly faster prediction of parameters like wall shear stress and velocity fields directly from aneurysm geometry. This approach utilizes machine learning models trained on existing CFD data to establish a relationship between geometric features and resulting hemodynamics, bypassing the need for iterative solving of Navier-Stokes equations during prediction. The resulting models can provide estimations in a fraction of the time required for traditional CFD, facilitating large-scale studies and potentially real-time clinical applications. While accuracy remains a key focus, current research aims to minimize the discrepancy between deep learning predictions and high-fidelity CFD results.
Multiple deep learning architectures are currently under investigation for the prediction of hemodynamic parameters. Convolutional Neural Networks (CNNs) are applied due to their established success in image processing, allowing them to analyze geometric representations of aneurysms. Graph Neural Networks (GNNs) are also being explored, as they can directly process mesh data, inherently capturing the relationships between nodes and elements within the aneurysm geometry. Finally, Transformer Architectures, originally developed for natural language processing, are being adapted to model spatial relationships and long-range dependencies within the complex flow fields, potentially offering improved accuracy and generalization capabilities compared to traditional methods.
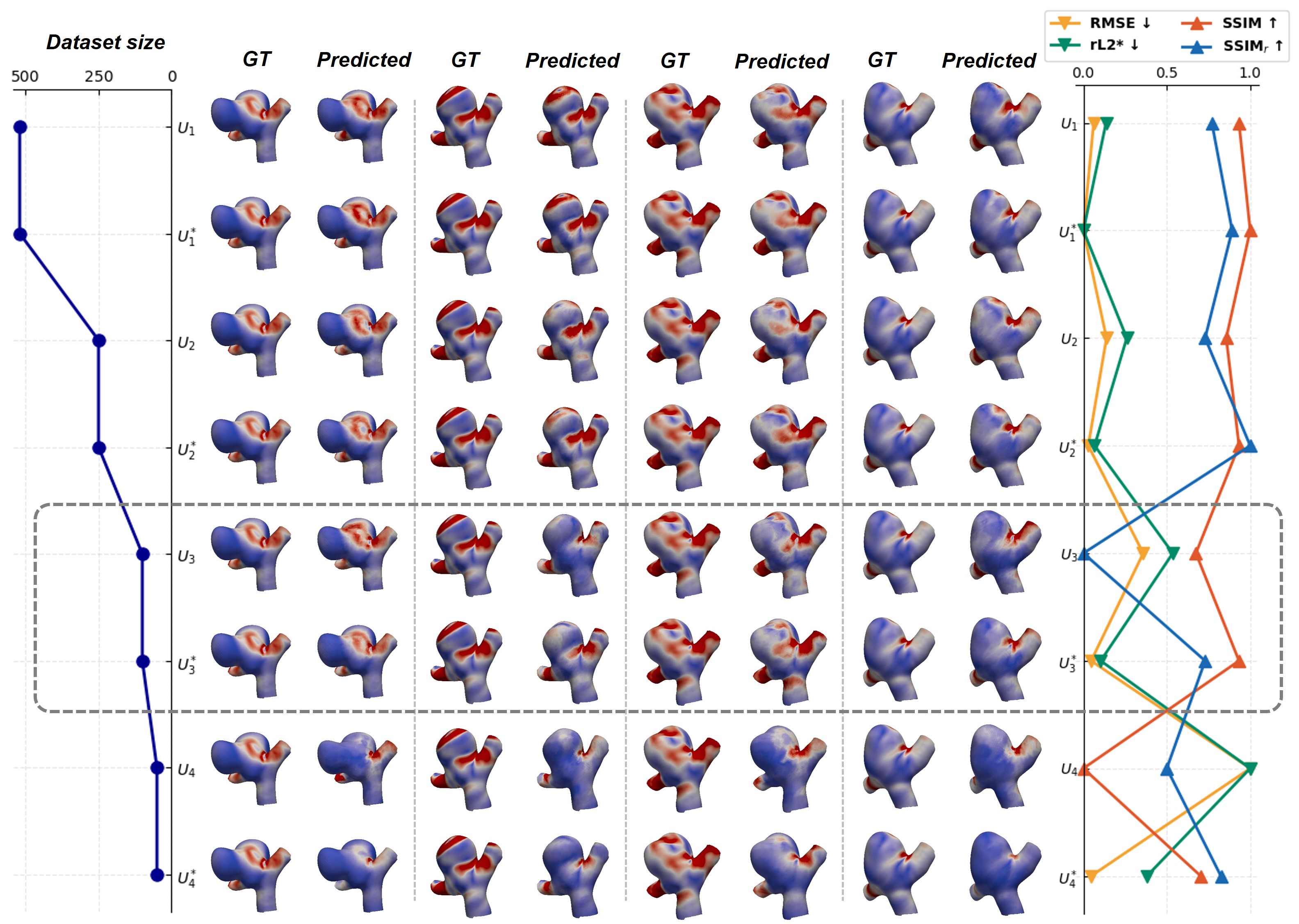
The AneuG-Flow Dataset is a publicly available resource consisting of computational fluid dynamics (CFD) simulations of 1,024 intracranial aneurysms. These simulations cover both steady and transient flow conditions, representing a range of aneurysm geometries and hemodynamic characteristics. The dataset includes high-resolution velocity and wall shear stress fields, serving as ground truth for training and validating deep learning models designed to predict hemodynamic parameters. Data is provided in a standardized format, facilitating model development and comparison across research groups. The inclusion of both steady and transient simulations is critical, as transient flow features are known to significantly influence aneurysm rupture risk, and accurately modeling these requires comprehensive training data.
Graph Isomorphism Networks (GINs) have been investigated for hemodynamic prediction, incorporating techniques to address challenges inherent in computational fluid dynamics (CFD) mesh variations. These networks utilize Graph Harmonic Deformation to standardize input meshes, enabling consistent analysis regardless of initial mesh density or topology. Further improving performance, a Global Attention Mechanism was implemented to allow the network to focus on the most relevant features within the aneurysm geometry. Evaluated on the AneuG-Flow dataset, this GIN architecture achieved a relative L2 error of 2.84\% (rL2*) when predicting hemodynamic parameters, indicating a high degree of accuracy compared to traditional CFD simulations.

The Inevitable Drift: Towards a More Nuanced Understanding
Rigorous evaluation confirms the deep learning models achieve high accuracy in predicting complex hemodynamic behavior and reconstructing detailed flow fields. Quantitative metrics demonstrate this precision: a Mean Squared Error (MSE) of 0.179 indicates a minimal average difference between predicted and actual values, while a Structural Similarity Index Measure (SSIM) of 0.967 confirms a strong perceptual similarity between reconstructed and ground truth flow patterns. These results validate the model’s ability to capture essential features of blood flow, offering a reliable foundation for its application in personalized cardiovascular assessments and treatment planning. The low MSE and high SSIM collectively establish the model as a robust tool for accurately simulating and analyzing fluid dynamics within the circulatory system.
The capacity to swiftly predict hemodynamic parameters promises a substantial refinement of clinical workflows. Traditional computational fluid dynamics (CFD) analysis, while accurate, is often time-consuming, creating a bottleneck in patient assessment. This deep learning approach offers a pathway to overcome this limitation, enabling clinicians to generate hemodynamic insights – such as blood flow velocity and wall shear stress – with unprecedented speed. Consequently, more patients can be evaluated in a timely manner, facilitating more accurate risk stratification for conditions like aneurysms or stenoses. Furthermore, the accelerated predictions empower personalized treatment planning, allowing physicians to tailor interventions – be they surgical, endovascular, or medical – to the specific hemodynamic profile of each patient, ultimately improving therapeutic efficacy and patient outcomes.
The deep learning models demonstrate enhanced predictive capabilities when trained with a technique called steady-data augmentation, a process particularly beneficial when access to comprehensive pulsatile flow data is restricted. This method strategically incorporates simulations of static, or steady-state, hemodynamics into the training dataset, effectively broadening the model’s exposure to diverse flow conditions. Consequently, the model learns to generalize more effectively from limited pulsatile data, improving its ability to accurately predict complex hemodynamic parameters and reconstruct detailed flow fields even with incomplete information. This approach not only bolsters the robustness of the deep learning framework but also addresses a common challenge in medical imaging, where acquiring sufficient pulsatile data can be both costly and time-consuming.
The successful implementation of this deep learning methodology promises a substantial reduction in the time and resources currently dedicated to computational fluid dynamics (CFD) analysis within clinical settings. Currently, detailed hemodynamic assessments rely heavily on manual CFD simulations, a process demanding significant expertise and computational power. By offering a rapid and accurate alternative for predicting blood flow and related parameters, this approach streamlines workflows, potentially enabling clinicians to evaluate a greater number of patient-specific cases. This increased efficiency isn’t merely about speed; it directly impacts patient care by facilitating more informed risk stratification and the development of truly personalized treatment plans, ultimately contributing to improved outcomes and a higher quality of life.
The pursuit of predictive modeling, as demonstrated in this work concerning intracranial aneurysms, echoes a fundamental truth about complex systems. One doesn’t construct a reliable forecast; rather, one cultivates the conditions for its emergence. The framework presented – a deep learning surrogate for computational fluid dynamics – isn’t a tool for controlling the prediction of wall shear stress, but an attempt to align with the inherent cyclicality of transient hemodynamics. As Blaise Pascal observed, ‘The eloquence of a man depends on his knowledge of the subject.’ This research, by deeply understanding the interplay of fluid dynamics and aneurysm geometry, strives for an eloquence of prediction – recognizing that even the most sophisticated model is merely a temporary accommodation within a larger, evolving system. Every dependency within the network is a promise made to the past, yet the system, inevitably, will begin fixing itself.
What Lies Ahead?
The pursuit of efficient hemodynamic surrogates for intracranial aneurysms, as demonstrated by this work, isn’t about solving a problem-it’s about carefully postponing a reckoning. The simplification inherent in any surrogate model-the reduction of a turbulent, time-varying flow to a manageable prediction-introduces a new class of error, a subtle divergence from the inevitable complexity of biological reality. It is not a question of accuracy, but of acceptable degradation over time. Architecture is, after all, how one postpones chaos.
The current focus on wall shear stress, while pragmatic, risks becoming a local maximum. Transient hemodynamics are not simply a function of surface geometry and flow rate; they are deeply entangled with vessel wall properties, pulsatile inflow conditions, and the aneurysm’s own evolving shape. Future work must grapple with the inherent limitations of relying solely on static meshes, perhaps integrating techniques that model wall distensibility or incorporate patient-specific arterial compliance. There are no best practices-only survivors.
Ultimately, the true challenge lies not in refining the surrogate itself, but in understanding the signal within the noise. Order is just cache between two outages. The pursuit of ever-more-realistic simulations may prove a distraction. The field would be better served by focusing on methods that can identify the critical parameters-the few variables that truly govern aneurysm rupture-even if those parameters remain stubbornly elusive.
Original article: https://arxiv.org/pdf/2601.19876.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- Lacari banned on Twitch & Kick after accidentally showing explicit files on notepad
- YouTuber streams himself 24/7 in total isolation for an entire year
- Ragnarok X Next Generation Class Tier List (January 2026)
- Answer to “A Swiss tradition that bubbles and melts” in Cookie Jam. Let’s solve this riddle!
- Gold Rate Forecast
- Best Doctor Who Comics (October 2025)
- 15 Lost Disney Movies That Will Never Be Released
- Best Zombie Movies (October 2025)
- 2026 Upcoming Games Release Schedule
- All Songs in Helluva Boss Season 2 Soundtrack Listed
2026-01-28 10:33