Author: Denis Avetisyan
Researchers have developed a novel framework that leverages causal reasoning and physiological knowledge to build ECG analysis models resilient to real-world variations and malicious attacks.

This work introduces CPR, a causal physiological representation learning method for robust and interpretable ECG analysis under distribution shifts, utilizing structural causal models and physiological priors.
Despite advances in deep learning for electrocardiogram (ECG) diagnosis, models remain vulnerable to subtle, biologically plausible perturbations, hindering reliable clinical application. This work introduces ‘CPR: Causal Physiological Representation Learning for Robust ECG Analysis under Distribution Shifts’, a novel framework that leverages physiological understanding and causal inference to disentangle robust pathological features from spurious correlations. By explicitly modeling ECG generation with a structural causal model, CPR achieves state-of-the-art robustness against adversarial attacks while maintaining single-pass inference efficiency-surpassing existing defenses in both performance and practicality. Could this approach pave the way for truly trustworthy and interpretable AI-driven cardiac monitoring?
Decoding the Signal: The Vulnerability of Modern ECG Analysis
Despite achieving remarkable accuracy in electrocardiogram (ECG) analysis, current deep learning models frequently operate on a foundation of empirical risk minimization (ERM), a process that prioritizes performance on the training dataset without necessarily capturing underlying physiological truths. This reliance on ERM creates a vulnerability to spurious correlations – the models learn to associate specific, but ultimately irrelevant, patterns in the ECG signal with particular diagnoses. Consequently, even subtle alterations to an ECG, unrelated to actual cardiac events, can mislead these systems, leading to incorrect interpretations. The models essentially learn ‘shortcuts’ rather than robust, biologically-plausible features, hindering their generalization to unseen data or in the presence of noise and signal variations common in real-world clinical settings. This dependence on superficial patterns, while allowing for high performance on curated datasets, raises concerns about the reliability and safety of these systems when deployed in practical healthcare applications.
Despite achieving remarkable accuracy, contemporary deep learning models analyzing electrocardiograms (ECGs) exhibit a significant vulnerability to adversarial attacks. These attacks involve subtly altering the input signal-in this case, the ECG-to induce misclassification, and increasingly, these alterations are designed to resemble natural biological variations. Smooth adversarial perturbations (SAP) are particularly concerning, as they introduce changes so minute and biologically plausible that they evade typical anomaly detection methods. Unlike readily identifiable noise, SAPs mimic the inherent complexities of a heart’s electrical activity, effectively camouflaging malicious intent within what appears to be a normal physiological signal. This makes defending against such attacks exceptionally challenging, as filtering or purification techniques risk removing genuine, albeit subtle, features of the ECG itself, potentially leading to false negatives and misdiagnosis.
Despite advancements in defending deep learning models used for electrocardiogram (ECG) analysis, current strategies like input purification and adversarial training (AT) frequently prove inadequate when confronted with subtle, yet meaningful, alterations to the ECG signal – known as semantic perturbations. These defenses often focus on pixel-level changes, failing to recognize manipulations that maintain biological plausibility but drastically alter the model’s interpretation. Beyond their limited efficacy, both input purification and AT demand substantial computational resources, significantly increasing the cost and complexity of deploying these systems in practical healthcare settings. This combination of vulnerability and expense creates a critical barrier to the reliable and widespread adoption of AI-powered ECG diagnostics, leaving a gap between research performance and real-world usability.
CPR: Reconstructing the ECG with Physiological Insight
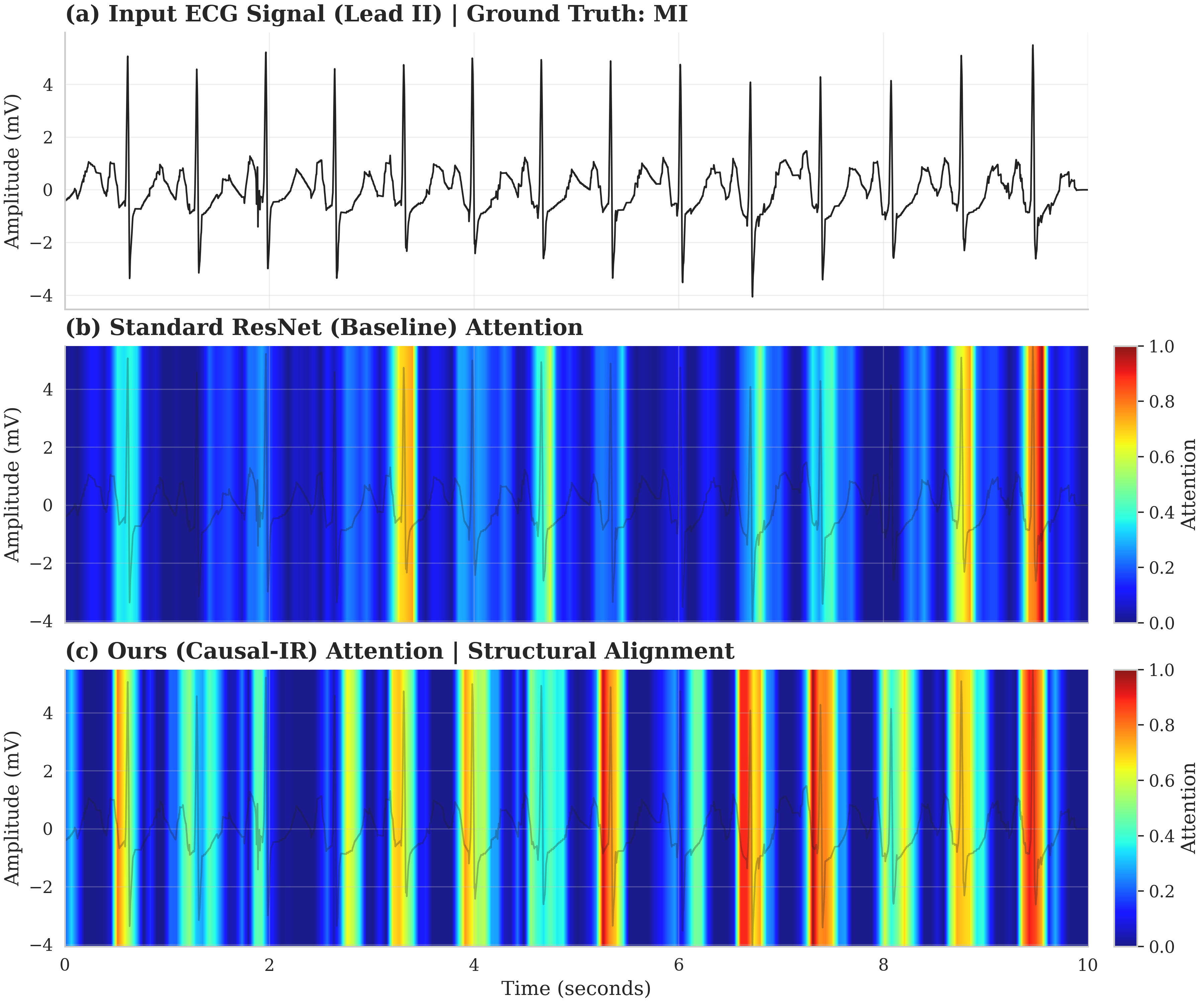
Current ECG analysis frequently relies on data-driven methodologies, such as deep learning, which can be susceptible to noise and lack interpretability. The CPR framework addresses these limitations by incorporating a priori physiological knowledge directly into the signal processing pipeline. This integration of structural priors – specifically, understanding the expected morphology of the P-QRS-T complex – allows CPR to move beyond purely correlational learning and model the underlying causal mechanisms generating the ECG signal. By explicitly representing the signal as a combination of pathological factors – representing disease – and style factors – encompassing noise and artifacts – CPR aims to create a more robust and interpretable analytical approach compared to traditional, purely data-driven methods.
The CPR framework utilizes a decomposition strategy to separate the observed ECG signal into two distinct latent factors: a pathological factor, Z_c, and a style factor, Z_s. Z_c is designed to represent the components of the signal directly attributable to underlying cardiac pathology, while Z_s encapsulates sources of variance unrelated to disease, such as patient movement, electrode contact issues, and baseline wander. This decomposition is not performed blindly; it is guided by a Physio-Mask, which provides anatomical and physiological constraints based on the expected morphology of the P-QRS-T complex. The Physio-Mask effectively directs the decomposition process to isolate disease-related signal changes from non-pathological variations, improving the accuracy and interpretability of downstream analysis.
The decomposition of the ECG signal into pathological and style factors is facilitated by employing separate Content and Style Encoders. The Content Encoder specifically focuses on extracting features from the P-QRS-T complex, utilizing known physiological constraints related to the timing and morphology of these waveforms. This encoder is designed to identify and isolate invariant pathological features – characteristics consistently associated with cardiac abnormalities, irrespective of noise or artifact. By prioritizing physiological plausibility within the encoding process, the system aims to reduce the impact of non-cardiac variations and enhance the robustness of feature extraction for diagnostic purposes. The Style Encoder, conversely, captures the remaining variance attributable to noise, artifacts, and individual patient characteristics, allowing for a clear separation of disease-related signal from confounding factors.
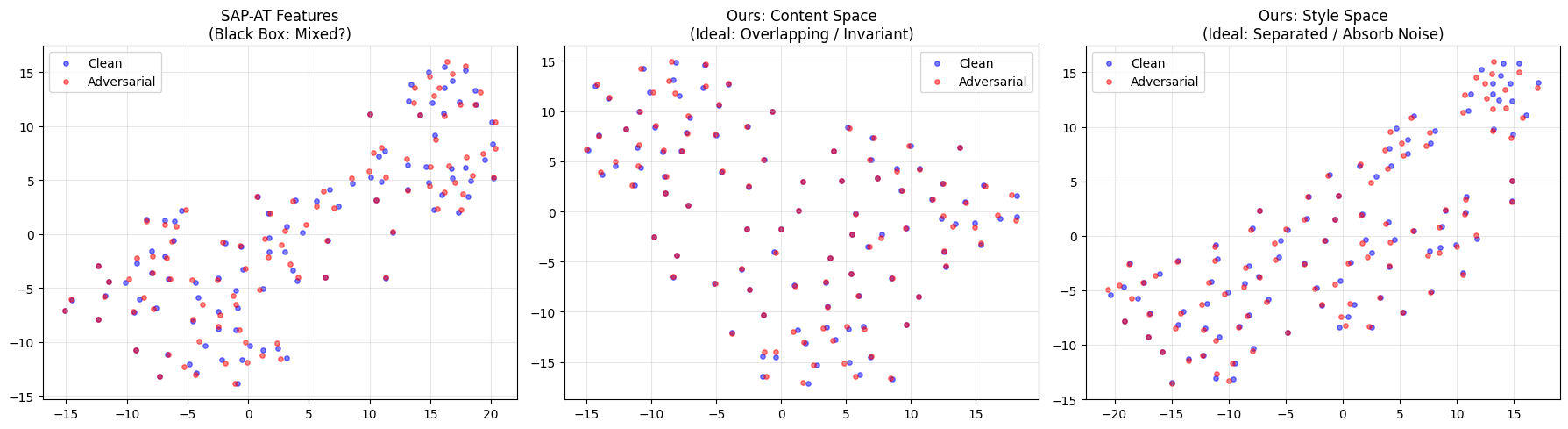
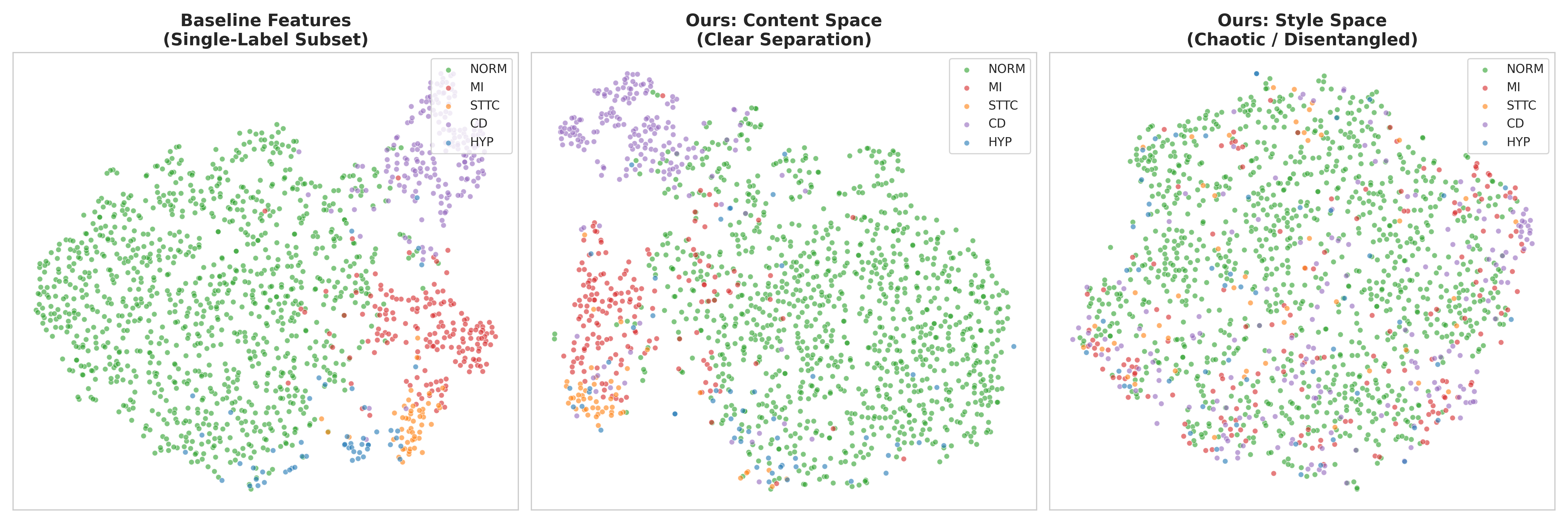
The CPR framework achieves a robust and interpretable latent space by mathematically enforcing independence between the pathological factor (Zc) and the style factor (Zs). This is accomplished through independence regularization techniques, which minimize statistical dependence between the two latent variables. Furthermore, semantic consistency techniques are employed to ensure that variations in Zc correspond to meaningful physiological changes, and that Zs primarily captures noise and artifacts. These constraints effectively disentangle the disease component from confounding factors, resulting in a latent space where similar pathologies cluster together and are readily distinguishable from variations due to noise or patient-specific characteristics. This disentanglement facilitates improved interpretability and allows for more accurate downstream tasks, such as disease classification and anomaly detection.
Stress-Testing the System: Robustness Against Adversarial Assaults
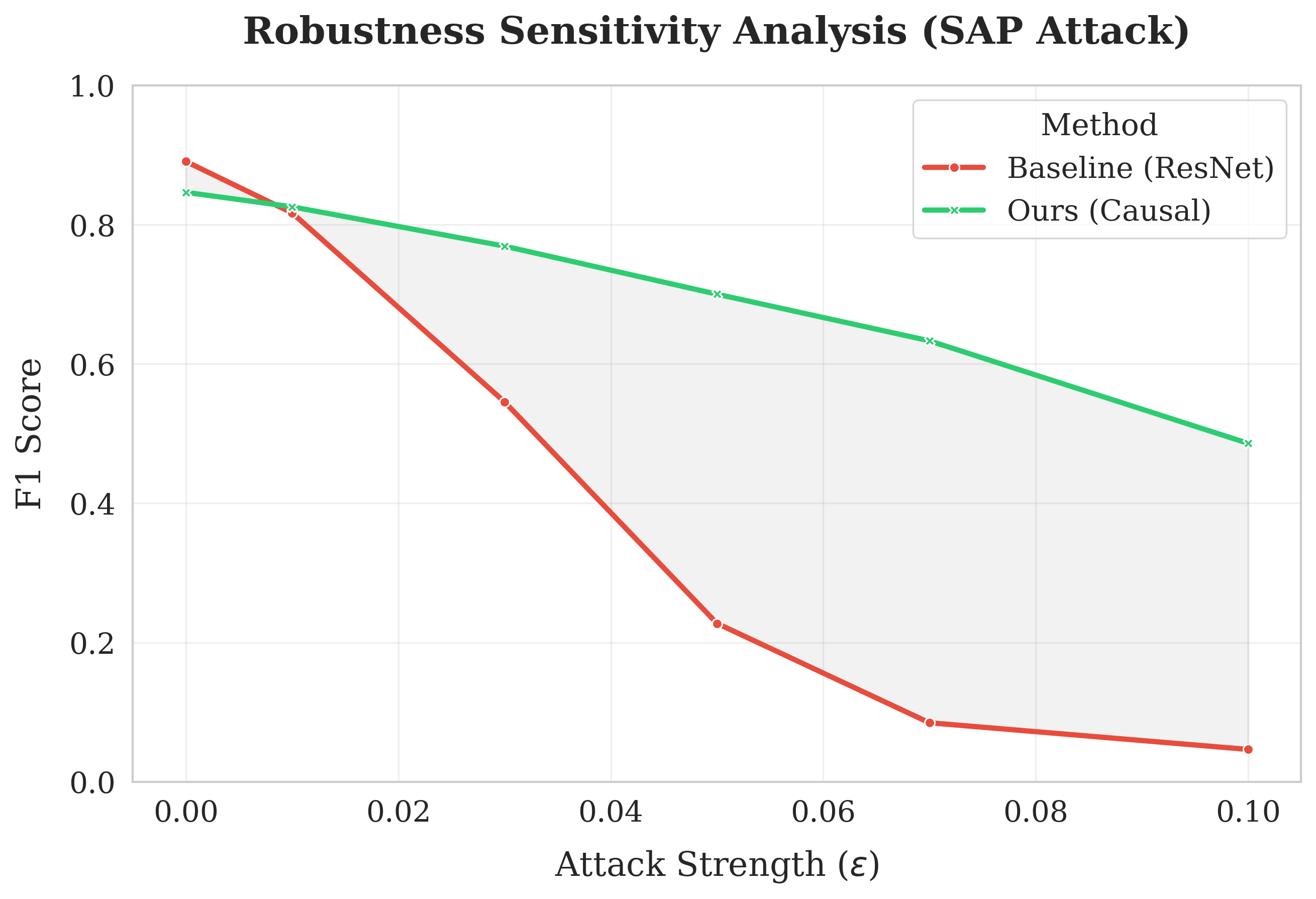
Evaluation of the CPR model on the PTB-XL dataset, a large electrocardiogram (ECG) database, indicates successful preservation of clinically relevant information even when subjected to adversarial perturbations. This was determined through quantitative analysis of key performance metrics, demonstrating CPR’s capacity to maintain diagnostic accuracy in the presence of intentionally crafted input noise. Specifically, the model’s ability to correctly identify and classify cardiac events was assessed both with and without adversarial attacks, confirming its resilience and utility in potentially compromised environments. The PTB-XL dataset provides a robust benchmark for evaluating the model’s performance given its size and clinical diversity.
Comparative analysis demonstrates that the proposed CPR model exhibits enhanced resilience against smooth adversarial perturbations (SAP). Specifically, CPR achieves an F1 score of 0.632 when subjected to SAP attacks, representing a substantial improvement over the CardioDefense model, which obtains an F1 score of 0.384 under identical attack conditions. This performance difference indicates a greater ability of CPR to maintain accurate predictions in the presence of subtle, intentionally crafted input distortions designed to mislead the model.
Evaluation of CPR on the Chapman-Shaoxing Dataset, a dataset separate from the training data, demonstrates its ability to generalize to new clinical data distributions without retraining. Under adversarial attack conditions, CPR achieved an F1 score of 0.437 on this dataset, representing a statistically significant improvement over the baseline model’s F1 score of 0.392. This result indicates that CPR’s performance is not limited to the specific characteristics of the PTB-XL dataset and can be maintained when applied to data with differing distributions, highlighting its robustness and adaptability in real-world clinical scenarios.
Clinical Physiological Reasoning (CPR) demonstrates increased resilience against adversarial feature invariance attacks due to its integration of established physiological principles. These priors constrain the model’s reliance on potentially manipulated or irrelevant features, as the reasoning process is grounded in known biological relationships. Unlike models susceptible to subtle, feature-level perturbations designed to induce misclassification, CPR leverages inherent physiological constraints to maintain accurate predictions even when input features are altered in ways that would deceive a purely data-driven approach. This dependence on biological plausibility effectively reduces the attack surface available to adversarial methods targeting feature invariance.
Beyond Prediction: Towards a Physiologically Grounded Future for ECG Analysis
The development of the Compositional Physiological Representation (CPR) framework marks a crucial advancement in electrocardiogram (ECG) analysis by moving beyond traditional, black-box machine learning approaches. Instead of solely relying on statistical correlations, CPR dissects the ECG signal into physiologically meaningful components – P wave, QRS complex, T wave – and models their relationships explicitly. This compositional approach not only enhances the robustness of the analysis, making it less susceptible to noise and artifacts, but also provides inherent interpretability; clinicians can understand why a particular diagnosis is being suggested, fostering trust and facilitating informed decision-making. By grounding AI in established physiological principles, the CPR framework establishes a pathway towards creating reliable clinical decision support systems capable of accurately identifying cardiac abnormalities and improving patient outcomes, while simultaneously offering a template for building similarly transparent and resilient AI tools across various medical specialties.
Ongoing investigations are poised to integrate increasingly sophisticated physiological models into the CPR framework, moving beyond simplified representations of cardiac function. This progression aims to capture the nuanced interplay of electrical and mechanical events within the heart, leading to a more accurate interpretation of ECG signals – particularly in the presence of noise or pathology. Simultaneously, researchers are exploring how CPR can be tailored to individual patient characteristics; by incorporating factors such as age, sex, body mass index, and pre-existing conditions, the system could deliver personalized analyses that account for inherent biological variability. This shift towards personalized ECG analysis promises to not only improve diagnostic accuracy but also to enable proactive risk stratification and the development of targeted interventions, ultimately enhancing patient outcomes.
The current framework for ECG analysis stands to gain substantial improvements through the integration of multi-modal data streams. Beyond the electrical signals captured by a standard electrocardiogram, a patient’s comprehensive medical history-including pre-existing conditions, lifestyle factors, and family history-offers crucial contextual information. Furthermore, incorporating genetic predispositions, particularly those linked to cardiac arrhythmias or structural heart diseases, could allow for proactive risk stratification and personalized diagnostic approaches. This holistic view, combining physiological signals with individual patient characteristics, promises to not only refine diagnostic accuracy but also to enhance predictive capabilities, potentially identifying individuals at risk before symptoms manifest and enabling tailored preventative strategies.
The core principles underpinning this ECG analysis framework extend far beyond cardiology, offering a valuable model for artificial intelligence development across diverse healthcare applications. Rather than relying on purely data-driven correlations, the emphasis on integrating established physiological understanding and prioritizing robust feature extraction – those directly linked to biological processes – promises to create AI systems less susceptible to spurious correlations and more adaptable to variations in patient data. This approach fosters resilience, allowing these systems to maintain accuracy even when confronted with noisy or incomplete information, a critical requirement for reliable clinical decision support in fields ranging from radiology and pathology to genomics and drug discovery. Ultimately, this blueprint advocates for a shift towards AI that isn’t simply ‘smart’ but fundamentally understandable and trustworthy, enhancing its utility and facilitating broader clinical acceptance.
The pursuit of robust ECG analysis, as detailed in this work, echoes a fundamental principle of understanding any complex system: dissection to reveal its underlying structure. It’s not enough to simply observe the signal; one must interrogate its causal mechanisms to build a model impervious to superficial distortions. This aligns perfectly with Donald Knuth’s assertion: “Premature optimization is the root of all evil.” The CPR framework, by prioritizing physiological priors and causal inference, deliberately avoids the trap of optimizing for mere predictive accuracy. Instead, it seeks a deeper, more principled understanding of the ECG signal, building a foundation for true resilience against distribution shifts and adversarial attacks – a system confessed its design sins when perturbed, and CPR attempts to fix them.
Beyond the Signal
The framework presented here-CPR-functions as a targeted exploit of comprehension. It doesn’t merely predict ECG characteristics; it attempts to disassemble the underlying physiological processes, a necessary, if arrogant, step towards true robustness. However, the current instantiation remains tethered to a pre-defined structural causal model. The real challenge isn’t building a prior, but constructing a system capable of autonomously reverse-engineering them from data-a dynamic prior, if you will. This necessitates a move beyond supervised disentanglement; the future lies in unsupervised discovery of genuinely independent physiological factors.
Furthermore, the resilience demonstrated against specific adversarial attacks shouldn’t be mistaken for comprehensive immunity. The landscape of potential perturbations is vast, and any model built on discernible patterns is, in principle, vulnerable. The next iteration must incorporate active, generative adversarial strategies-not just defense against known exploits, but proactive probing for unforeseen weaknesses.
Ultimately, the pursuit of robust ECG analysis isn’t about achieving perfect prediction. It’s about building a system that admits its own ignorance, continuously refining its internal model of causality. The signal is merely the messenger; the true target is the mechanism itself, and that requires a willingness to dismantle, to question, to break things in the name of understanding.
Original article: https://arxiv.org/pdf/2512.24564.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- Abiotic Factor Update: Hotfix 1.2.0.23023 Brings Big Changes
- Gold Rate Forecast
- I’m Convinced The Avengers: Doomsday Trailers Are Using The Same Trick As Infinity War
- Answer to “Hard, chewy, sticky, sweet” question in Cookie Jam
- Silver Rate Forecast
- Brent Oil Forecast
- Katanire’s Yae Miko Cosplay: Genshin Impact Masterpiece
- Top gainers and losers
- Should You Use DLC Personas in Persona 5 Royal? The Great Debate
- Why Doggos Are the Ultimate Collectible in Abiotic Factor
2026-01-03 21:37